Successful biosynthesis of the precursors of powerful anticancer drug vinblastine in yeast
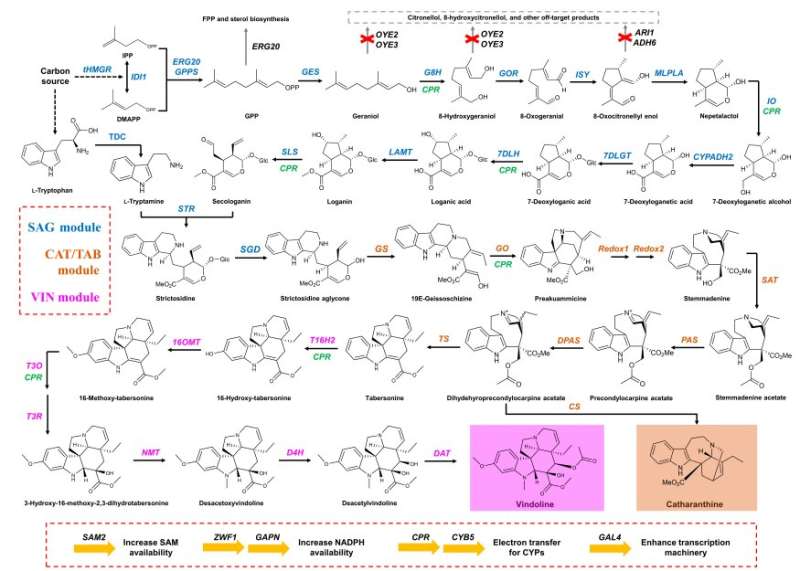
Humanity’s fight against cancer is expected to continue for years to come. Scientists across the globe are constantly inventing novel synthetic methods for the rapid and efficient production of anticancer therapeutics. However, the chemical synthesis of one such powerful anticancer drug—vinblastine—poses major challenges, owing to its complex three-dimensional structure. Vinblastine is a heterodimer of the chemical compounds catharanthine and vindoline.
Unfortunately, currently available methods for the mass production of vinblastine are both costly and unsustainable.
Recently, a research team from Zhejiang University, China, led by Dr. Jiazhang Lian and Dr. Di Gao, made a breakthrough by identifying a novel method for the biological synthesis of catharanthine and vindoline—the two pharmacologic precursors of vinblastine. It has previously been noted that the biosynthesis of these two precursors occurs via a 31-step-long reaction in Catharanthus roseus—a medicinal plant also known for its ornamental value.
Drawing inspiration from this study, the research team genetically engineered Saccharomyces cerevisiae, commonly known as yeast cells, and utilized them to produce vindoline and catharanthine. The chemical/biological coupling of vindoline and catharanthine was then used to obtain vinblastine. These findings were published in BioDesign Research.
Dr. Lian, who serves as a Principal Investigator at Zhejiang University’s College of Chemical and Biological Engineering, remarks, “On the basis of a platform strain with a sufficient supply of precursors and cofactors for biosynthesis, we could successfully optimize the biosynthetic pathways to produce vindoline and catharanthine. Using shake flask fermentation, our engineered yeast strains were able to produce catharanthine and vindoline at a titer of 527.1 μg/L and 305.1 μg/L, respectively, without accumulating detectable amount of pathway intermediates.”
To modify the genome of the yeast cells, the team used the popular “CRISPR-Cas9” technique, further tweaking the technique to get rid of certain production bottlenecks. For instance, introducing an extra copy of the genes that coded for certain key enzymes significantly boosted the overall yield of catharanthine from 225.3 μg/L to 527.1 μg/L.
Similarly, when the team realized that the production of tabersonine—a reaction intermediate that ultimately gets converted to vindoline—was lower than that of catharanthine, they swiftly removed this bottleneck by introducing extra copies of genes coding for the associated enzymes. This increased the yield of tabersonine from 9.0 μg/L to 18.9 μg/L. A similar strategy was used to increase the yield of vindoline.
Discussing the future implications of these findings, an ecstatic Dr. Lian adds, “The production of vinblastine using microbial cell factories holds great promise for its large-scale and cost-effective production. In fact, it can act as a representative example for the production of additional valuable plant products in yeast.”
In summary, although the biosynthesis of vindoline and catharanthine involves one of the most complicated pathways ever reconstituted in yeast-based cell factories, the research team was able to achieve this with advanced genetic engineering and synthetic biology-based approaches.
More information:
Di Gao et al, De Novo Biosynthesis of Vindoline and Catharanthine in Saccharomyces cerevisiae, BioDesign Research (2022). DOI: 10.34133/bdr.0002
Provided by
BioDesign Research
Citation:
Successful biosynthesis of the precursors of powerful anticancer drug vinblastine in yeast (2023, January 11)
retrieved 12 January 2023
from https://phys.org/news/2023-01-successful-biosynthesis-precursors-powerful-anticancer.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

