Sinhcaf-dependent histone deacetylation essential for primordial germ cell specification
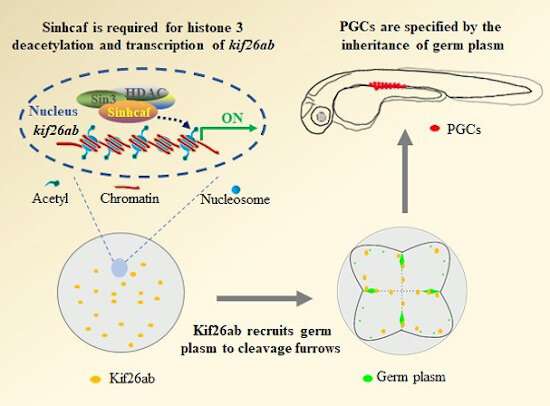
Primordial germ cells (PGCs) are the first germ-cell population established during development. The survival of species is dependent upon PGCs in sexually reproducing organisms because they are the founder cells for the germline.
In Caenorhabditis elegans, Drosophila melanogaster, Xenopus laevis and the zebrafish Danio rerio, PGCs are specified by inheritance of maternal germ plasm during early embryogenesis. Germ plasm is both sufficient and necessary for zebrafish PGC specification, and germ plasm accumulation at cleavage furrows requires coordination of microtubules, actin cytoskeletons and molecular motors. However, how this process is regulated remains poorly understood.
Recently, a research team led by Prof. Hu Wei from the Institute of hydrobiology (IHB) of the Chinese Academy of Sciences provides the first evidence that Sinhcaf-dependent histone deacetylation is essential for germ plasm aggregation and primordial germ cell specification. The study was published in EMBO Reports.
The researchers first generated sinhcaf knockout and overexpression zebrafish lines. They found that the maternal-zygotic sinhcaf mutants (MZsinhcaf -/-) exhibited germ plasm aggregation defects, decreased PGC abundance and male biased sex ratio. All of these effects were rescued by sinhcaf overexpression which led to excess PGCs and a female-biased sex ratio.
Using co-immunoprecipitation analysis, the researchers found that zebrafish Sinhcaf interacted with the core components of SIN3-HDAC complex. Western blot results indicated that loss of Sinhcaf resulted in an increased acetylation level of histone 3 in zebrafish full-grown stage (FG) follicles.
The researchers then conducted transcriptomic and real-time quantitative PCR analysis, and revealed that the level of kif26ab transcripts was significantly decreased in sinhcaf mutant FG follicles and mature eggs. Chromatin immunoprecipitation results further demonstrated Sinhcaf can directly bind to the transcriptional regulatory region of kif26ab.
Loss of sinhcaf increased both acetyl-histone H3 (K9) and acetyl-histone H3 (K18) levels in the promoter region of kif26ab, said Prof. Hu, adding that kif26ab is transcriptionally activated by Sinhcaf, according to the luciferase-promoter assay.
The researchers demonstrated that suppressed expression of kif26ab caused defective germ plasm aggregation in cleavage furrows, and decreased PGCs number in genital ridge. Injection of kif26ab mRNA could partly rescue the decreased germ plasm components in cleavage furrows of MZsinhcaf -/- embryos.
This study uncovers a critical role of Sinhcaf in germ plasm aggregation and subsequent PGC specification, which is mediated by regulating the histone acetylation status of kif26ab promoter to activate its transcription. The sinhcaf -/- mutants offer an amendable in vivo model system to determine what controls PGC specification.
X marks the spot: How genes on the sex chromosomes are controlled
Binbin Tao et al, Sinhcaf‐dependent histone deacetylation is essential for primordial germ cell specification, EMBO reports (2022). DOI: 10.15252/embr.202154387
Citation:
Sinhcaf-dependent histone deacetylation essential for primordial germ cell specification (2022, May 10)
retrieved 11 May 2022
from https://phys.org/news/2022-05-sinhcaf-dependent-histone-deacetylation-essential-primordial.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

