Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly
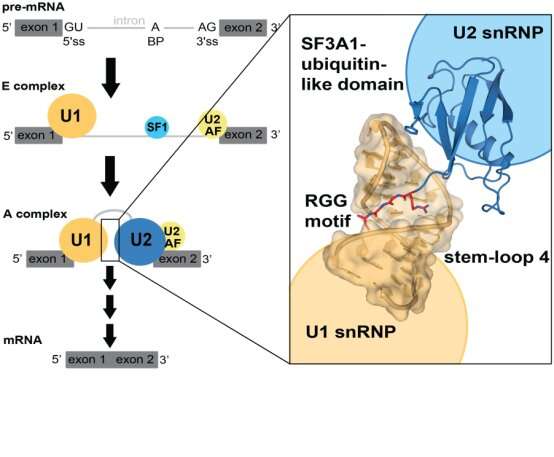
A recent collaborative PNAS paper by the Allain (IBC), Jonas (IMBB), Leitner (IMSB) and Sharma groups (University of Arizona) reveals how early spliceosome complexes are brought together via specific RNA-binding of an unstructured arginine-glycine-glycine (RGG) motif.
Pre-messenger RNA (pre-mRNA) splicing is a key regulatory step in gene expression. Malfunctioning of splicing causes several neurological diseases, such as spinal muscular atrophy (SMA) or amyotrophic lateral sclerosis (ALS). The splicing reaction is mediated by the spliceosome, a dynamic complex comprising five small nuclear ribonucleoproteins (snRNPs), which assembles onto each intron in multiple steps. During the early steps of spliceosome assembly, pre-mRNA bound U2 snRNP interacts with U1 snRNP for functional positioning of critical splicing elements. Thus far, this step has not been structurally characterized in mammals, even though it is a crucial step for splicing regulation.
In this study, the authors determined the 3D structure of the ubiquitin-like RNA-binding domain of U2 snRNP protein SF3A1 in complex with a stem-loop RNA of U1 snRNP. Crystallography, NMR spectroscopy, and cross-linking mass spectrometry revealed that the ubiquitin-like domain of SF3A1 recognizes sequence-specifically the RNA target. An RGG-motif of SF3A1 helps reading the RNA stem sequence via the arginine side chain and the main chain of both glycines.
The study is the first structural characterization of an ubiquitin-like domain and broadens the functional scope of unstructured RGG/RG-rich motifs in RNA binding proteins. Furthermore, the study provides a molecular basis of an early step of spliceosome assembly, which may help develop innovative therapeutic strategies against diseases originating from splicing defects.
Discovery of a subset of human short introns spliced out by a distinct mechanism
Tebbe de Vries et al, Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2114092119
Citation:
Sequence-specific RNA recognition by an RGG motif connects U1 and U2 snRNP for spliceosome assembly (2022, February 3)
retrieved 3 February 2022
from https://phys.org/news/2022-02-sequence-specific-rna-recognition-rgg-motif.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

