Researchers use fluorine-doping method to construct catalysts with enhanced performance
As industry has developed over the past century, excess carbon dioxide emission had led to climate problems and greenhouse effects. Scientists are constantly working toward solutions for the problems of greenhouse gases, which are warming the earth’s surface and the lower parts of the atmosphere. Carbon dioxide is the most prevalent of the greenhouse gases.
Carbon dioxide can be electrochemically reduced into valuable chemicals using wind or sunlight-derived electrical energies. This carbon dioxide electroreduction presents scientists with a promising strategy for managing the carbon balance on a global scale. Electrochemical reduction of carbon dioxide offers the future potential for converting the carbon dioxide into useful, more environmentally friendly chemicals, such as carbon monoxide, methane, or ethanol. To achieve carbon dioxide electroreduction, scientists need efficient electrocatalysts. Electrocatalysts are the catalysts used in electrochemical reactions. They can increase the rate of the reaction that occurs. A research team from Nanjing University has constructed catalysts using a fluorine-doping method that enhances their performance.
The research team reported their findings in Nano Research.
Scientists know that low-cost metal-nitrogen-carbon single-site catalysts work well for carbon dioxide electroreduction to carbon monoxide. Among these, the nickel-nitrogen-doped carbon single-site catalysts possess the high faradaic efficiency of carbon monoxide and large partial current. The faradaic efficiency describes how efficiently charge is transferred in an electrochemical reaction.
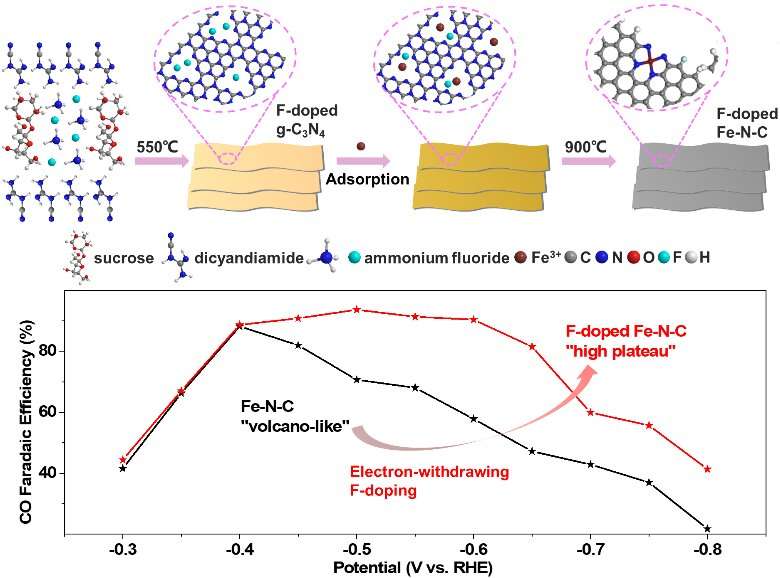
The research team has already boosted the faradaic efficiency and large partial current of nickel-nitrogen-doped carbon single-site catalysts by doping them. By comparison with the nickel-nitrogen-doped carbon single-site catalysts, iron-nitrogen-carbon single-site catalysts have lower overpotentials for carbon dioxide electroreduction. Overpotential describes a cell’s voltage efficiency. Previous research has used X-ray absorption fine structure spectroscopy to verify that the active sites of the iron-nitrogen-carbon single-site catalysts are Fe3+ sites. These Fe3+ sites allow the catalyst to be more effective in carbon dioxide adsorption and carbon monoxide desorption.
The team constructed a fluorine-doped iron-nitrogen-carbon single-site catalyst which possesses more Fe3+ sites, as they expected. The fluorine-doped iron-nitrogen-carbon single-site catalyst they constructed maintained the advantage of low overpotential. It also promoted the carbon monoxide faradaic efficiency from a volcano-like high value to a high plateau value. “The results indicate the superior stability of fluorine-doped iron-nitrogen-carbon to iron-nitrogen-carbon because of the fluorine-doping,” said Lijun Yang, an associate professor from School of Chemistry and Chemical Engineering, Nanjing University.
The research team concludes that the electron-withdrawing fluorine-doping allows the iron-nitrogen-carbon single-site catalyst to maintain the advantage of low overpotential, with a much increased carbon monoxide faradaic efficiency and partial current density because of the stabilized Fe3+ sites.
The team synthesized the iron-nitrogen-carbon using a heat method called adsorption pyrolysis. They performed the carbon dioxide electroreduction experiments in an H-type cell and a gas diffusion electrode cell. They used theoretical calculations to further understand the improvements that happened with the fluorine doping.
“Electrochemical tests show the enriched defects by fluorine-doping kinetically increase the electroactive surface area and improve charge transfer,” said Yang. Looking ahead to further study, the research team’s findings provide them with a simple and controllable strategy to enhance the carbon dioxide electroreduction to carbon monoxide performance of iron-nitrogen-carbon catalysts by stabilizing the Fe3+ sites.
Tandem catalyst to enhance carbon dioxide electroreduction to methane
Yiqun Chen et al, Boosting faradaic efficiency of CO2 electroreduction to CO for Fe−N−C single-site catalysts by stabilizing Fe3+ sites via F-doping, Nano Research (2022). DOI: 10.1007/s12274-022-4441-0
Provided by
Tsinghua University Press
Citation:
Researchers use fluorine-doping method to construct catalysts with enhanced performance (2022, June 17)
retrieved 17 June 2022
from https://phys.org/news/2022-06-fluorine-doping-method-catalysts.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

