Researchers identify novel human-specific mechanism of skin barrier regeneration
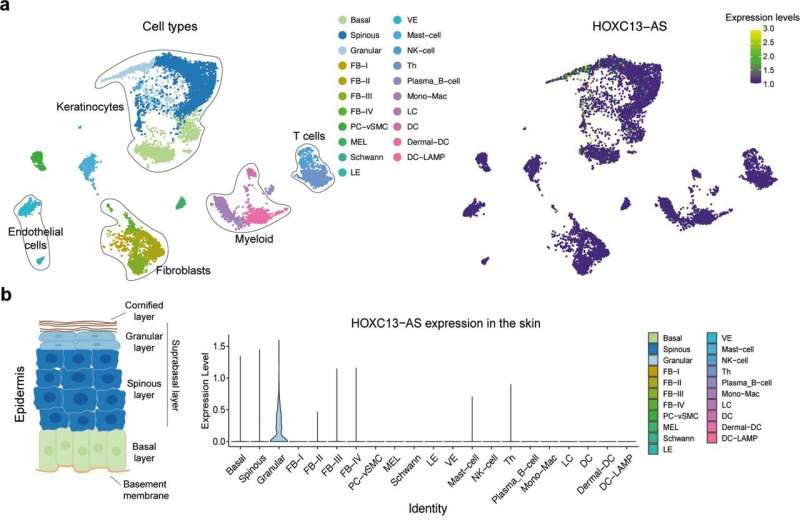
Researchers at Karolinska Institutet, Department of Medicine, Solna, have identified HOXC13-AS, a human skin-specific long noncoding RNA (lncRNA), that plays a crucial role in epidermal differentiation. Their study highlighting lncRNA’s physiological importance in the epidermal barrier’s maintenance and reconstruction is published in the journal Cell Death & Differentiation.
When the skin gets injured, the epidermal keratinocytes switch their states from homeostasis to regeneration to reconstruct the epidermal barrier. However, the dynamic gene expression and regulatory mechanisms underpinning this switch are enigmatic. Such knowledge is required to understand the pathological mechanism underlying failed re-epithelization in chronic non-healing wounds. Although not coding for proteins, many lncRNAs have been identified as important regulators in fundamental biological processes, but their role in epidermal differentiation remains largely unexplored.
In the new study, researchers from Karolinska Institutet performed RNA sequencing in human skin and wounds and identified an lncRNA, HOXC13-AS, specifically expressed in human skin and its expression was significantly downregulated when the skin gets injured. The study reveals that HOXC13-AS promotes keratinocyte differentiation, and is thus crucial for the epidermal barrier function. Mechanistically, HOXC13-AS interacts with COPA (COPI Coat Complex Subunit Alpha) proteins that participate in the retrograde transport of cargo proteins from the Golgi to the endoplasmic reticulum (ER).
“Our study demonstrates that the interaction between HOXC13-AS and COPA proteins interferes with the retrograde transport, which leads to ER stress and keratinocyte differentiation,” says the first author, Dr. Letian Zhang, at the Department of Medicine Solna, who successfully defended his Ph.D. recently.
“The specific expression in human skin and the critical function in regulating ER stress make HOXC13-AS a potential therapeutic target for a range of cutaneous diseases showing chronic ER stress,” says Ning Xu Landén, Senior Lecturer at the Department of Medicine, Solna, who is the PI leading this study.
More information:
Letian Zhang et al, Human skin specific long noncoding RNA HOXC13-AS regulates epidermal differentiation by interfering with Golgi-ER retrograde transport, Cell Death & Differentiation (2023). DOI: 10.1038/s41418-023-01142-z
Citation:
Researchers identify novel human-specific mechanism of skin barrier regeneration (2023, March 22)
retrieved 22 March 2023
from https://phys.org/news/2023-03-human-specific-mechanism-skin-barrier-regeneration.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

