Researchers find HERC1 protein deficiency causes osteopenia
Bones remain healthy thanks to the fact that they are continuously remodeling, a process dependent on the balance between the activity of osteoblasts—cells that create bone tissue—and the osteoclasts, which reabsorb it. An imbalance between these two can disrupt bone homeostasis and lead to diseases such as osteopenia, which is a loss of bone mass.
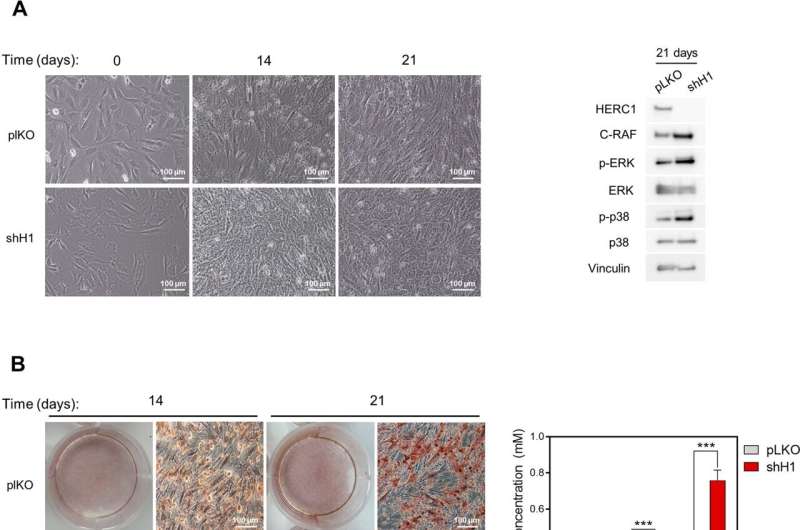
Now, a study carried out on model animals and published in the journal Cell Death & Disease reveals how a HERC1 protein deficiency builds the first evidence of the role of HERC1 protein in bone homeostasis. The study has been carried out by the research group Cell Signaling and Bone Biology of the University of Barcelona and the Bellvitge Biomedical Research Institute (IDIBELL).
The study explains how the HERC1 deficit stimulates both the activity of osteoblasts and osteoclasts, a process that favors bone reabsorption rather than bone formation and which leads to osteopenia in adulthood.
The conclusions of the study show that, in young mice, female individuals with HERC1 deficiency are more likely to develop osteopenia, and this is correlated to lower levels of testosterone and dihydrotestosterone.
Osteoblasts and osteocytes also synthetize RANKL and OPG proteins, which regulate in the opposite way from the differentiation and activation of osteoclasts. During the development of osteopenia generated by HERC1 deficiency, an increase in the RANKL/OPG ratio and in the number of osteoclasts is observed. These results identify RANKL as a potential therapeutic target for individuals with bone affectations and HERC1 deficiency.
According to Jose Luis Rosa, professor at the UB’s Faculty of Medicine and Health Sciences, head of the IDIBELL group and coordinator of the study, “the early detection of HERC1 variants with loss of function could identify individuals with predisposition to develop osteopenia and other pathologies related to bone metabolism, which could improve their treatment and medical evolution.”
More information:
Leonardo Pedrazza et al, HERC1 deficiency causes osteopenia through transcriptional program dysregulation during bone remodeling, Cell Death & Disease (2023). DOI: 10.1038/s41419-023-05549-x
Citation:
Researchers find HERC1 protein deficiency causes osteopenia (2023, March 3)
retrieved 3 March 2023
from https://phys.org/news/2023-03-herc1-protein-deficiency-osteopenia.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

