Researchers fabricate cobalt copper catalysts for methane on metal-organic framework
The world is highly dependent on fossil fuels to power its industry and transportation. These fossil fuels lead to excessive carbon dioxide emission, which contributes to global warming and ocean acidification. One way to reduce this excessive carbon dioxide emission that is harmful to the environment is through the electroreduction of carbon dioxide into value-added fuels or chemicals using renewable energy. The idea of using this technology to produce methane has attracted wide interest. However, researchers have had limited success in developing efficient catalysts for methane.
A Soochow University research team has now developed a simple strategy for creating cobalt copper alloy catalysts that deliver outstanding methane activity and selectivity in electrocatalytic carbon dioxide reduction. Their research is published in Nano Research.
Over the past 10 years, scientists have made notable progress in advancing their understanding of catalysts and applying the knowledge to their fabrication. But the catalysts that have been developed have not been satisfactory for use with methane, in terms of selectivity or current density. Despite the great insights scientists have gained, the strategies they have attempted in creating catalysts for methane are just too costly to be useful in practical applications.
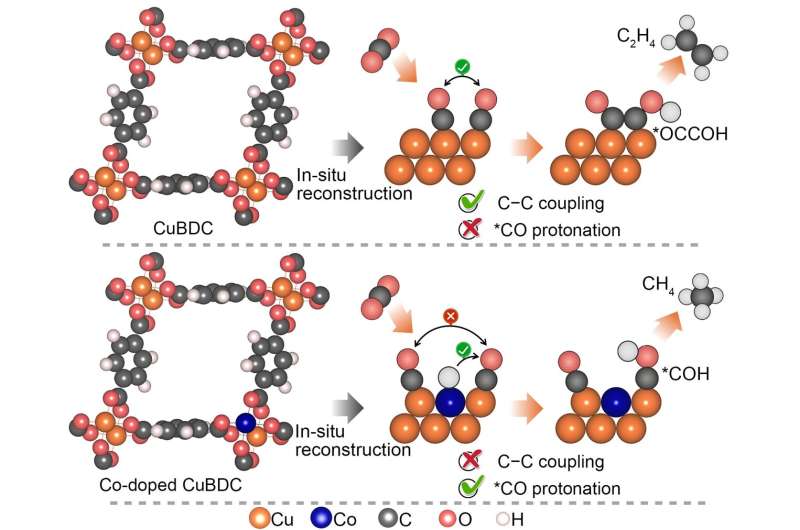
The Soochow University team looked to metal organic frameworks as a way to overcome the earlier challenges in constructing catalysts for methane. “The metal organic frameworks have been perceived as a unique category of electrochemical carbon dioxide reduction reaction catalyst since they offer a tunable platform to systematically alter the metal site coordination, regulate the Helmholtz layer, and control over the intermediates binding,” said Professor Yang Peng, Soochow Institute of Energy and Materials Innovations, College of Energy, Soochow University. The Helmholtz layer refers to the boundary or interface that appears where an electronic conductor comes in contact with an ionic conductor.
Yet the stability of metal organic frameworks during the electrolytic process remains a limiting issue. So metal organic frameworks are often used as the structural precursor to derive more robust catalyst ensembles upon reconstruction. In their research, the team took advantage of the metal organic framework’s homogenously dispersed metal centers. They attained electrochemically reduced cobalt copper alloys that deliver outstanding methane activity and selectivity in electrocatalytic carbon dioxide reduction. The team used in-situ X-ray adsorption spectroscopy and attenuated-total-reflection surface enhanced infrared spectroscopy in the development of their strategy.
The team’s study not only offers a useful strategy for constructing electrocatalytic carbon dioxide reduction catalysts through the electrochemical reconstruction of bimetallic metal organic frameworks, but also furnishes important insights into the steering of electrocatalytic carbon dioxide reduction pathways on copper via atomic doping of 3d transition metals. These 3d transition metals are the elements on the periodic table running from 22Ti to 29Cu (titanium to copper).
By modulating the cobalt doping concentration, the team achieved a remarkable Faradaic efficiency of 60% to methane at a high operating current density.
“The most important message we would like to deliver in this work is that by atomically doping other 3d transition metals in to copper, even in a small quantity, the electrocatalytic carbon dioxide reduction energetics and pathway can be controllably modulated,” said Peng.
As a next step, the team wants to achieve better stability. They will do this by testing the catalytic system in a membrane electrode assembly. “Our ultimate goal is to achieve industrial-scale productivity and stability of methane production and realize the resourceful utilization of carbon dioxide in a green fashion,” said Peng.
Review of technologies that boost potential for carbon dioxide conversion to useful products
Hao Sun et al, Atomically dispersed Co−Cu alloy reconstructed from metal-organic framework to promote electrochemical CO2 methanation, Nano Research (2022). DOI: 10.1007/s12274-022-4728-1
Provided by
Tsinghua University Press
Citation:
Researchers fabricate cobalt copper catalysts for methane on metal-organic framework (2022, August 12)
retrieved 12 August 2022
from https://phys.org/news/2022-08-fabricate-cobalt-copper-catalysts-methane.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

