Researchers develop high-adsorption phosphates for radionuclide cesium ion capture
Nuclear energy is crucial for producing cleaner energy, but the associated radioactive pollution requires strategic solutions. Cesium (Cs+) is a toxic radionuclide generated from nuclear power plants that demands immobilization and high adsorption methods to prevent environmental pollution.
Although phosphate-based adsorbents are excellent candidates for cleanup, their inefficient ion exchange leads to limited adsorption capacity. The high theoretical adsorption of phosphate adsorbents does not match their experimental adsorption capacities.
To remove harmful Cs+ from radioactive wastewater, Pusan National University researchers led by Professor Kuk Cho from the Department of Environmental Engineering have synthesized dittmarite-type phosphates with a layered structure, ideal for easy ion exchange.
The team found that their magnesium phosphates had record-high adsorption capacities for Cs+, surpassing standard adsorbents due to exchangeable ions and dissolution-precipitation. Prof. Cho says, “The presence of exchangeable ions and dissolution-precipitation enabled record-high adsorption capacities for Cs+ that are higher than those of standard adsorbents.”
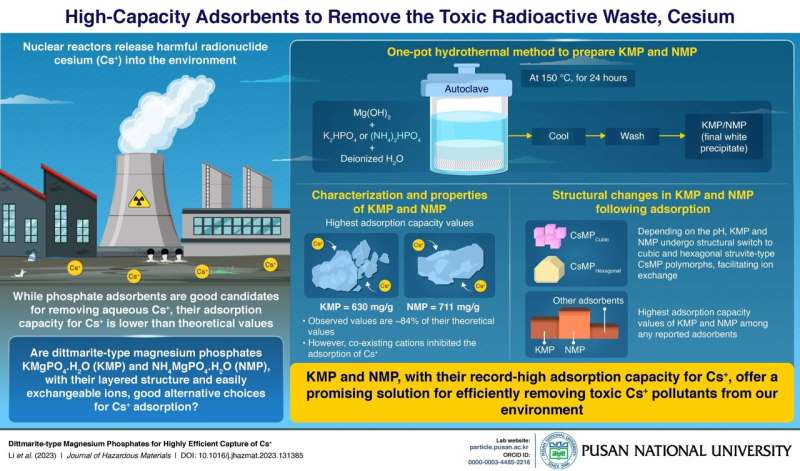
The study was published in the Journal of Hazardous Materials. Using a one-pot hydrothermal method, the team synthesized KMgPO4⋅H2O (KMP) and NH4MgPO4⋅H2O (NMP), both of which are dittmarite-type compounds, having a high theoretical adsorption capacity of 754 mg g− 1 and 856 mg g− 1 for Cs+, respectively.
The synthesized KMP and NMP had remarkable adsorption capacities of 630 mg g−1 and 711 mg g−1, respectively, which were 84% of their theoretical adsorption capacities. These experimentally measured adsorption capacity values are the highest among all reported adsorbents for Cs+.
Next, the team characterized and analyzed the physical and chemical properties of the phosphates. Based on the Cs+ adsorption performance of KMP and NMP, they showed that these phosphates are not best suited for use in water with high divalent ion concentrations. However, they can still be used in Cs+ readsorption processes, following desorption processes, to concentrate Cs+ and reduce waste volume.
Prof. Cho says, “Cs+ is a popular radionuclide generated from nuclear power plants, and the volume of its waste must be minimized for disposal. To minimize the volume, the adsorbent with higher adsorption capacity is advantageous.”
The study found that the new phosphates efficiently adsorb Cs+, providing a cost-effective method for radioactive waste disposal. This is particularly important in a world where nuclear power plants are expected to increase in number, and proper storage with appropriate adsorbents will become crucial for sustainability.
In conclusion, the high adsorption capacities and stability of the synthesized phosphates make them promising candidates to deal with the radioactive waste disposal challenge.
More information:
Zeqiu Li et al, Dittmarite-type magnesium phosphates for highly efficient capture of Cs+, Journal of Hazardous Materials (2023). DOI: 10.1016/j.jhazmat.2023.131385
Provided by
Pusan National University
Citation:
Researchers develop high-adsorption phosphates for radionuclide cesium ion capture (2023, May 4)
retrieved 4 May 2023
from https://phys.org/news/2023-05-high-adsorption-phosphates-radionuclide-cesium-ion.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

