Ray of hope for mitochondrial diseases: Discovery of a protein’s surprising second role could aid diagnosis
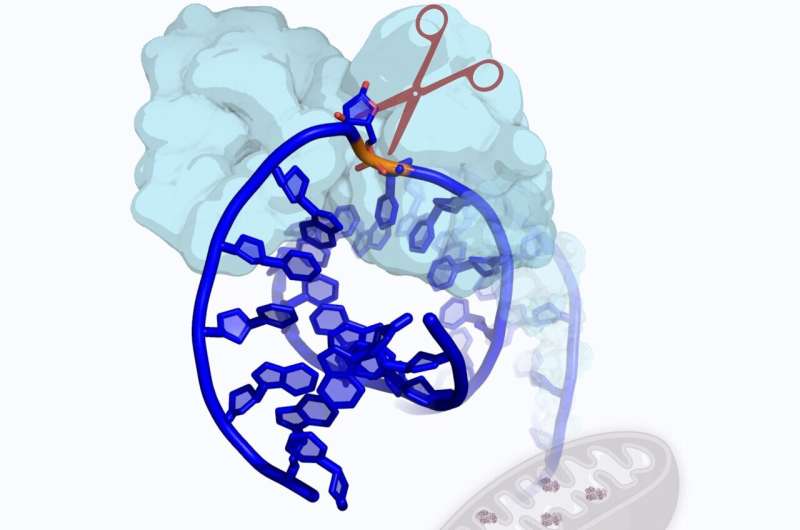
A protein that packs massive DNA strands into tiny cells also moonlights as a cleaner of damaged genetic material. This discovery could help detect mitochondrial diseases, which can cause brain damage and organ failure.
DNA carries all the information about how a living organism will look and function. In human cells, most of it is found in the nucleus. However, some DNA is also found in the mitochondria, a small organelle known as the “powerhouse of the cell” because it is responsible for converting food and oxygen into life-sustaining energy.
If mitochondria cannot create energy or send signals to the rest of the cell, cell injury and even cell death can follow. If this process is repeated throughout the body, entire organs stop working as they should.
“Mitochondrial DNA is less studied, even though it has been linked to cancer, aging, and these mitochondrial diseases which can affect every aspect of the body,” said Linlin Zhao, an assistant professor of chemistry at UC Riverside.
Zhao’s laboratory examines the ways that mitochondria repair or remove bits of DNA molecules that have been damaged by chemicals from inside the body or external, environmental factors. He and his colleagues learned through a series of studies that one protein in particular, TFAM, facilitates this cleansing process.
A paper describing this discovery has been published in the journal ACS Chemical Biology and featured as a cover article. Zhao said he was surprised by the findings, because TFAM has traditionally been known for having completely different functions.
“One of the main recognized roles of this protein is packaging DNA into the mitochondria,” Zhao said. “DNA can stretch several thousand times longer, in length, than the region of the mitochondria where it resides. TFAM helps wrap up the DNA so it can fit.”
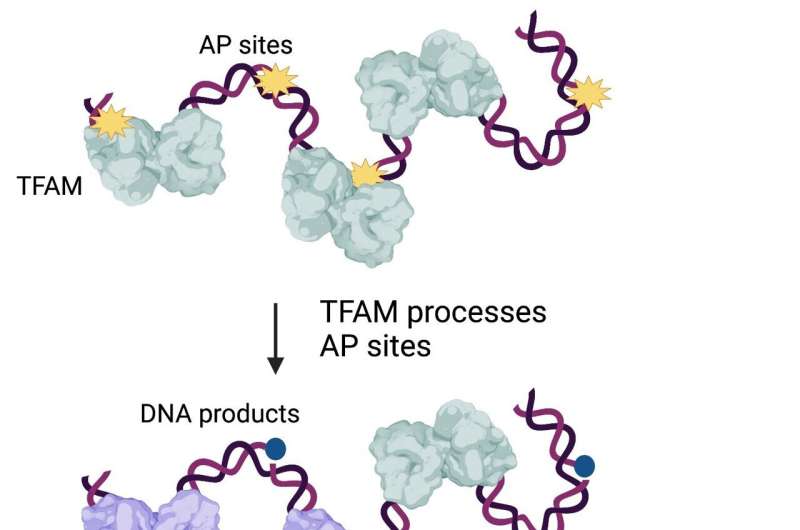
The research team observed that when TFAM encounters lesions in the DNA called AP sites, it behaves like molecular scissors and cuts the damaged DNA. It also forms additional molecules that signal to the cell it needs to further break down damaged genetic material and eliminate the unhealthy molecules.
DNA damage is common. The scientific literature estimates that as many as 100,000 AP sites form inside each cell in a person’s body every day, in the mitochondria as well as the nucleus.
“TFAM molecules bound to AP sites amplify signals that tell mitochondria, “This DNA is no longer good; go copy other, healthier molecules,'” Zhao said.
This study also provides chemical insight into which building blocks or amino acids of the TFAM protein kickstart the process that allows it to break down damage at AP sites. Gaining insight into the ways that mitochondria function can help clinicians understand more about what’s happening when they aren’t functioning as they should.
Testing for levels of TFAM that are attached to DNA, or for the DNA products that result from TFAM processing AP sites, might help physicians diagnose mitochondrial health. This would be a significant boost to people suffering from mitochondrial diseases. While there is currently no cure for this kind of disease, there are treatments that help manage symptoms and slow disease progression.
“As people study more, it’s becoming clear that mitochondrial diseases are not so rare as once thought. But they are poorly diagnosed because the symptoms often overlap with those of other diseases, making them tough to recognize,” Zhao said. “Anything we can do to improve detection, so treatment can be offered earlier, would really be beneficial.”
More information:
Wenxin Zhao et al, Key Amino Acid Residues of Mitochondrial Transcription Factor A Synergize with Abasic (AP) Site Dynamics To Facilitate AP-Lyase Reactions, ACS Chemical Biology (2023). DOI: 10.1021/acschembio.3c00047
Citation:
Ray of hope for mitochondrial diseases: Discovery of a protein’s surprising second role could aid diagnosis (2023, July 26)
retrieved 26 July 2023
from https://phys.org/news/2023-07-ray-mitochondrial-diseases-discovery-protein.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

