pH-activated nanocytokines based on IL-12 safely overcome cancer resistance to immunotherapy
A group led by Prof. Horacio Cabral, in collaboration with a group led by Prof. Kazuhiro Kakimi, discovered a new way to improve cancer immunotherapy. Their findings are published in a paper, titled “An IL-12-Based Nanocytokine Safely Potentiates Anticancer Immunity through Spatiotemporal Control of Inflammation to Eradicate Advanced Cold Tumors,” in Advanced Science.
Many solid tumors have immunosuppressive microenvironment, which prevents the infiltration, activation, and effector function of immune cells, resulting an immunologically cold tumor phenotype that is insensitive to immunotherapy conducted by immune checkpoint inhibitors (ICIs), including the famous anti-PD-1 and anti-CTLA-4 antibodies.
Interleukin-12 (IL-12) is one of the strongest pro-inflammatory cytokines. Thus, there is a major interest to use it for enhancing the immunity in tumors and improving the response rates of immunotherapies. However, IL-12 is extremely toxic due to systemic immune activation and shows limited clinical efficacy under safe dose. To solve such problem, here an IL-12-based nanocytokine that can control the immune activation function of IL-12 based on intratumoral pH were developed to realize a tumor-targeted immunity enhancement.
Based on pH-sensitive polymer materials, the nanocytokine can sense the pH in tumors to unleash the fully active cytokine. As a result, the nanocytokine avoids the systemic response mediated by IL-12 and diminishes the side effects. In murine triple-negative breast cancer (TNBC) model, the nanocytokine enhances the immunity and elevates the immune cells’ infiltration level in the tumor. The nanocytokine also infuses potency to ICIs, completely eradicating both primary and metastatic tumors.
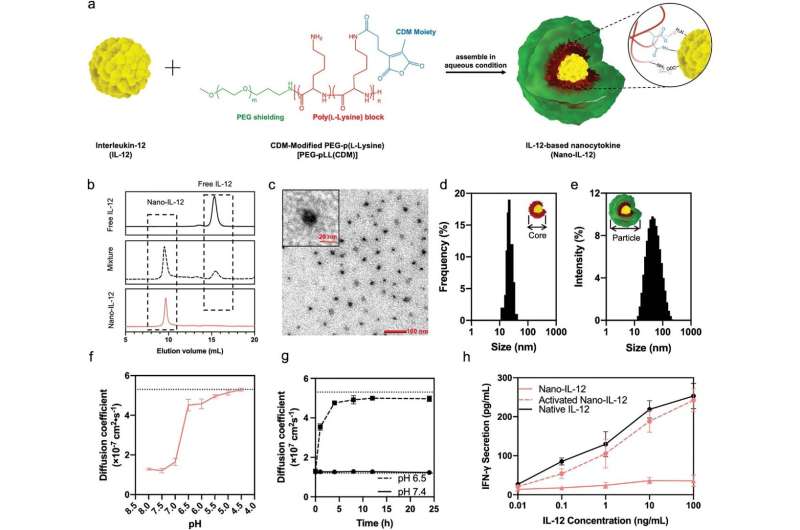
What is the novelty of this study?
IL-12 has long been investigated as potential anti-cancer agents but its strong toxicity and insufficient therapeutic efficacy under maximum tolerated dose were illustrated by early-stage clinical trials and called for strategies to solve such problem before its application. The nanocytokine formulation is the first example of polymer-based switchable IL-12 delivery system, and in the results of this study, it was found that:
- A polymer-based nanocytokine formulation can stably control the “off” and “on” state of IL-12’s bioactivity by sensing the pH difference between healthy organs and tumor.
- The tumor-targeted activation of the nanocytokine leads to spatiotemporal control of the immune reactions mediated by IL-12. In healthy tissues, the off-target immune activation and counteractive reactions are diminished, meanwhile in tumor, the inflammatory responses are enhanced.
- The nanocytokine profoundly inflames cold tumors from the gene expression level and synergize with ICIs to completely eradicate advanced primary and metastatic tumors.
Why are these findings important and how is the study going to improve the current therapy?
Interleukin-12 (IL-12) is one of the strongest pro-inflammatory cytokines. Thus, there is a major interest to use it for inflaming tumors and increasing the response rates of immunotherapies. Nevertheless, IL-12 is extremely toxic due to systemic immune activation, and shows limited clinical efficacy, even at its maximum tolerated dose (MTD), because its spatiotemporal inflammatory dynamics trigger counteracting immune reactions.
While big efforts are being dedicated to engineer IL-12 protein for increasing its safety and tumor selectivity, these approaches fall short in overcoming IL-12’s issues, as they could still induce systemic inflammation, promote interfering anti-inflammatory signals and, in many cases, present lower equimolar activity than native IL-12. Unfortunately, the inflammatory dynamics for such protein-engineered systems are still poorly understood, and the promotion of counteractive secondary responses remains unclear.
The IL-12-based nanocytokine solves the said issues at once by promoting the spatiotemporal control of the inflammation through high tumor accumulation, high and selective intratumoral immune activity and regulated interaction with the systemic immunity. Thus, nanocytokine controlled the inflammation is a spatiotemporal manner.
It blocked the systemic immune response and avoided the onset of adaptive anti-inflammatory signals after repeated injection, e.g., the expansion of interleukin-10 (IL-10), which are commonly observed after the second injection of IL-12 or standard IL-12-based systems, such as fusion proteins and immunocytokines. Such spatiotemporal control could be advantageous for realizing repeated administration schedules without the declining of the anti-tumor effects.
The nanocytokine also expanded the therapeutic window of IL-12 through the spatiotemporal control of inflammation. Thus, the nanocytokine benefitted from the blocking of counteractive immune responses to decrease by 10-fold the effective dose against immunosuppressive triple negative breast cancer. Moreover, the nanocytokine enhanced the safety, and was non-toxic even at a dose that is 1,000-fold higher than the reported MTD of native IL-12 in human studies.
The nanocytokine profoundly inflamed immunosuppressive tumors, avoiding the stimulation of immune responses that interfere with efficacy. This allowed the nanocytokine to strongly synergize with immune checkpoint inhibitors (ICIs) by reversing the immunosuppressive tumor microenvironment.
Notably, because the nanocytokine is based on biocompatible FDA-approved materials, the strategy holds high potential for clinical translation. In addition, the formulation could be extended to other therapeutic proteins, serving as a practical strategy for tumor-targeted delivery of biologics.
More information:
Pengwen Chen et al, An IL‐12‐Based Nanocytokine Safely Potentiates Anticancer Immunity through Spatiotemporal Control of Inflammation to Eradicate Advanced Cold Tumors, Advanced Science (2023). DOI: 10.1002/advs.202205139
Provided by
Innovation Center of NanoMedicine
Citation:
pH-activated nanocytokines based on IL-12 safely overcome cancer resistance to immunotherapy (2023, February 7)
retrieved 7 February 2023
from https://phys.org/news/2023-02-ph-activated-nanocytokines-based-il-safely.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

