Novel spider silk materials can be optimized to produce cell-specific effects
Materials made of spider silk can be specifically modified or processed in such a way that living cells of a certain type adhere to them, grow and proliferate. This has been discovered by researchers at the University of Bayreuth under the direction of Prof. Dr. Thomas Scheibel.
Cell-specific effects of the materials can be generated by biochemical modifications of the silk proteins, but also by surface structuring of spider silk coatings. The research findings, published in Advanced Healthcare Materials and Advanced Materials Interfaces, are pioneering for regenerative medicine and the production of artificial tissue.
Spider silk promotes the formation of natural tissue in a cell-specific manner
In many cases, biomedical restoration of damaged or destroyed tissue depends on stimulating and controlling the development of specific cells. Cells of different types, for example skin, muscle and nerve cells, must be involved in order to create a functioning cell network. A scaffold of spider silk implanted in the body, to which a growing number of newly developing cells attach, provides important prerequisites for this natural rebuilding process: Spider silk proteins are biodegradable and generally compatible with existing cells of the organism.
The Bayreuth research results obtained at the Chair of Biomaterials now show how such a scaffold made of spider silk can be optimized. For spatially different sections of the scaffold, materials can be used in the future that are particularly well suited for the targeted attachment, growth and proliferation of cells of a required cell type.
As a result, such a spider silk scaffold implanted in the body is ideally suited for the production of large natural tissue structures involving different cell types. It is gradually degraded in a natural way as the tissue regeneration progresses.
Spider silk implant coatings suppress rejection reactions
The results of the two studies will also benefit the optimization of implants that are intended to permanently replace natural tissue and remain in the body. This requires materials that ensure that the implants are not rejected by inflammation or allergic reactions.
A coating of spider silk, which is optimally adapted to the respective cell types in the surrounding tissue and promotes their attachment, helps to avoid such rejection reactions and thus contributes to the smooth integration of the implant into the natural tissue.
Cell-specific effects through biochemical modifications
As the Bayreuth researchers have demonstrated, cell-specific effects of spider silk materials can be produced by functionally modifying silk proteins through the incorporation of peptides, which are short-chain polyamino acids. Peptides that interact with cells (cell-adhesive peptides) are present in the extracellular matrix (ECM) of natural tissues: This is a lattice-like molecular structure that fills the spaces between neighboring cells in a tissue and stabilizes their spatial arrangement.
The Bayreuth researchers have grafted some cell-adhesive peptides found in the ECM of numerous organisms—including humans—into several variants of a silk protein derived from a spider silk of the garden cross spider. As a result of biochemical modification, some of these altered silk proteins were found to be generally cell-adhesive, while others generally exhibited cell-repellent behavior.
In some cases, however, cell-specific interactions were observed in addition. Particularly striking were the effects of the peptide KGD: it specifically promotes the attachment and growth of myoblasts. These are embryonic muscle precursor cells that can develop into muscle fibers.
“Our research results point to a novel pathway leading to cell-specific applications of materials made from spider silk—whether in the design of scaffolds to promote natural regenerative processes, in the coating of implants, or even in the 3D printing of hydrogels with encapsulated cells that can be further processed into functional materials,” said Vanessa Trossmann M.Sc., lead author of the study published in Advanced Healthcare Materials.
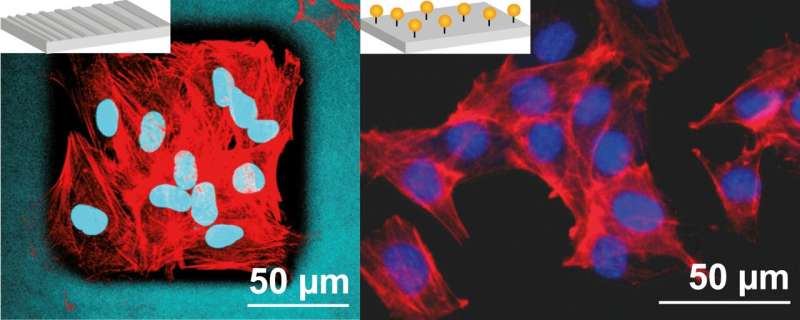
Cell-specific effects through surface structuring of spider silk coatings
The study, published in Advanced Materials Interfaces, presents a different way to optimize spider silk materials. Coatings made from one silk protein derived directly from the silk of the garden spider do not exhibit cell-adhesive behavior on their own—without biochemical modification.
The research team led by Prof. Dr. Thomas Scheibel has now used a lithographic process to structure the surface of these coatings in such a way as to specifically stimulate the attachment and growth of cells of a certain type. The reactions of different cells to the shape and size of the geometric structures imprinted in the surface depend strongly on the respective cell type, among other parameters, as extensive tests have shown.
“Based on our research results, it will be possible to lithographically optimize coatings made of silk proteins or other biocompatible materials in such a way that they stimulate and drive the natural regeneration of complex tissue structures in a cell-specific manner,” says Scheibel.
More information:
Vanessa Tanja Trossmann et al, Design of Recombinant Spider Silk Proteins for Cell Type Specific Binding, Advanced Healthcare Materials (2022). DOI: 10.1002/adhm.202202660
Sarah Lentz et al, Selective Topography Directed Cell Adhesion on Spider Silk Surfaces, Advanced Materials Interfaces (2022). DOI: 10.1002/admi.202201936
Citation:
Novel spider silk materials can be optimized to produce cell-specific effects (2023, May 3)
retrieved 3 May 2023
from https://phys.org/news/2023-05-spider-silk-materials-optimized-cell-specific.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

