New hybrid catalyst could help decarbonization and make ethylene production more sustainable
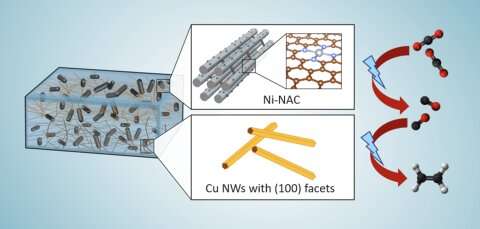
A new hybrid catalyst converts carbon dioxide into ethylene in one pot. The catalyst was developed by scientists from Ames National Laboratory, Iowa State University, University of Virginia, and Columbia University. This catalyst supports the world net-zero carbon initiative by using carbon dioxide (CO2) as a feedstock for efficient ethylene production powered by electricity.
Ethylene is a commodity chemical used to manufacture a wide range of products from plastics to antifreeze. The large-scale production of ethylene is energy intensive and relies heavily on fossil resources. Electrocatalytic production of ethylene from CO2 is emerging as a promising method. This new catalyst consists of only earth-abundant materials, such as nickel and copper, and requires less energy for chemical reaction.
Long Qi, a scientist at Ames Lab, explained how the catalyst works. Atomically dispersed nickel anchored on nitrogen assembly carbon (NAC) works to catalyze CO2 to CO at low voltage and high current. The catalyst is effective over a wide range of voltages and its effectiveness at higher currents means a higher rate of CO production.
“Since this catalyst remains active over a very wide voltage range, that allows easy coupling with a second catalyst,” Qi said. “So we use the second catalyst, which is a copper nanowire, and by combining these two we have a very selective process that has up to 60% efficiency going from CO2 to ethylene in one pot.”
Another important aspect of the catalyst is its structure. Wenyu Huang, an Ames Lab scientist and Iowa State University professor from the team, noted that the catalyst’s porous structure enhances its effectiveness. “Our catalyst has an ordered mesoporous structure that is beneficiary for mass transfer,” he said. “Because it’s highly porous, you have a very high surface area to expose a lot of nickel’s active sites, making our catalyst very effective in CO2 reduction to CO.”
For Huang, the most exciting aspect of this research was how the team combined the two catalysts to streamline the process. “We basically combine the two best catalysts on their own, and they work together so we can connect the CO2 to CO and the CO to ethylene reactions in one system,” he said.
Qi emphasized the importance of using CO2 as a feedstock for this reaction, because it addresses the global need to reduce the amount of CO2 released into the atmosphere. He explained that this process can use CO2 recovered from chemical or industrial processes, or from air capture. “And we can do this without any precious metal, simply the nickel, copper, carbon, and nitrogen, to permit large-scale industrial applications,” Qi said. “Also, we potentially eliminate the use of fossil resources to make ethylene.”
This research is further discussed in the paper “Hybrid Catalyst Coupling Single-Atom Ni and Nanoscale Cu for Efficient CO2 Electroreduction to Ethylene,” published in the Journal of the American Chemical Society.
More information:
Zhouyang Yin et al, Hybrid Catalyst Coupling Single-Atom Ni and Nanoscale Cu for Efficient CO2 Electroreduction to Ethylene, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c09773
Citation:
New hybrid catalyst could help decarbonization and make ethylene production more sustainable (2023, January 23)
retrieved 23 January 2023
from https://phys.org/news/2023-01-hybrid-catalyst-decarbonization-ethylene-production.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

