Maximizing extracellular vesicles: A new protocol on isolation and quantification to optimize patient care
It’s a big step forward in using extracellular vesicles (EVs) as a diagnostic tool. Lead study author, J. Nathaniel Diehl, Ph.D., at the UNC School of Medicine, led research showing how a new protocol in handling extracellular vesicles can improve significant diagnostic and therapeutic potential in disease development.
Extracellular vesicles (EVs) are fragments of cells that break off and circulate through the body. The importance of their role in the body has come into clearer focus in the last few years, whereas previously researchers had no idea if these vesicles had any real function or if they were just “cellular debris.” It is now known that EVs exert their biological functions by delivering proteins, metabolites, and nucleic acids to recipient cells. They also play important roles in disease development, such as pancreatic cancer, multiple myeloma, or possibly Marfan syndrome—an inherited disorder that affects connective tissue.
One study published in PLOS ONE titled, “A Standardized Method For Plasma Extracellular Vesicle Isolation and Size Distribution Analysis,” led by first author, J. Nathaniel Diehl, Ph.D., a fourth-year medical student at the UNC School of Medicine, described the workflow for isolation and quantification of plasma EVs—a protocol to help labs in the field measure the quantity and size of EVs in the blood stream of patients.
“Our hope is that the protocol will allow our lab or others to build on this work to help identify patients who may need treatment without other invasive or expensive tests,” Diehl said. “Rather, they could have a small sample of their blood drawn, and the lab could test their blood for EVs. This technology is a long way from being available in the hospital or clinic. However, this protocol is a stepping stone for that technology,” he said.
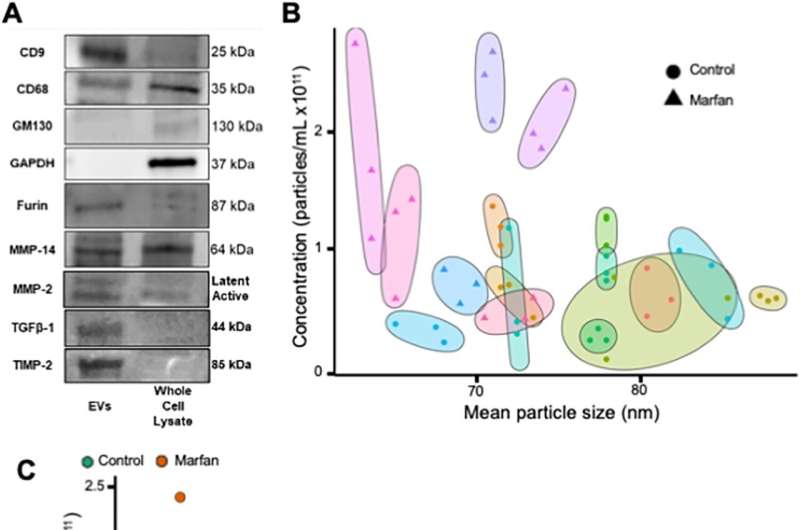
As a sample of the diagnostic potential, Diehl and his team of researchers focused on patients with Marfan syndrome with aortic aneurysms and showed that these patients have circulating EVs that are smaller and more abundant than healthy patients.
Results were also highly consistent across technical replicates (one person running the same sample multiple times) and across different personnel (two different people running the same sample). Diehl said these results gave them substantially more confidence in this new protocol’s technique and its potential applications.
“This work is a big step forward for using EVs as a possible diagnostic tool in the clinic,” said Diehl. “Our team defined and streamlined a technique that can be used to answer any number of clinical questions involving circulating EVs.”
As a member of the under Adam W. Akerman, Ph.D. and John S. Ikonomidis, MD, Ph.D., at the UNC School of Medicine, Diehl and his team’s goal is to understand the underlying biology and pathology that leads to progression of aortic aneurysms, with a focus on patients with connective tissue disorders like Marfan syndrome. Their hope is to leverage this understanding to define methods for earlier diagnosis and possible treatments that may treat or prevent progression of aortic aneurysms.
“This work is a launching point for studying EVs and their role in this disease process,” said Diehl. “It’s an important step towards possibly developing a blood-based test for aortic aneurysm diagnosis or monitoring. While this field is still new and there is more work to be done, we could potentially see applications of this technique as a method of monitoring patients with known aneurysms in order to avoid expensive imaging.”
Diehl said that existing imaging techniques, like CT scans, provide substantial information about blood vessels and anatomic structures critical for diagnosing aneurysms. However, they also have downsides including radiation exposure, particularly when a patient requires routine tracking of the aneurysm. With this novel protocol, researchers are now able to outline a procedure starting from a blood draw all the way through analysis of the data. Diehl said currently available literature did not have any protocols or techniques that outlined best practices to this extent.
“It remains to be seen whether EVs contribute directly to the progression of aortic aneurysms,” said Diehl. “At a minimum, our work demonstrates that EVs circulate in the blood stream at a higher concentration and are smaller in patients with Marfan syndrome with aortic aneurysms. Future work from our lab and others will hopefully define the specific roles of signaling proteins and nucleic acids contained within EVs.”
More information:
J. Nathaniel Diehl et al, A standardized method for plasma extracellular vesicle isolation and size distribution analysis, PLOS ONE (2023). DOI: 10.1371/journal.pone.0284875
Citation:
Maximizing extracellular vesicles: A new protocol on isolation and quantification to optimize patient care (2023, May 8)
retrieved 8 May 2023
from https://phys.org/news/2023-05-maximizing-extracellular-vesicles-protocol-isolation.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

