Light-to-energy conversion in many aquatic microbes more complex than was previously known, researchers discover
Plants convert light into a form of energy that they can use—a molecule called adenosine triphosphate (ATP)—through photosynthesis. This is a complex process that also produces sugar, which the plant can use for energy later, and oxygen. Some bacteria that live in the light-exposed layers of water sources can also convert light to ATP, but the process they use is simpler and less efficient than photosynthesis. Nonetheless, Technion—Israel Institute of Technology researchers now find this process isn’t as straightforward and limited as was previously thought.
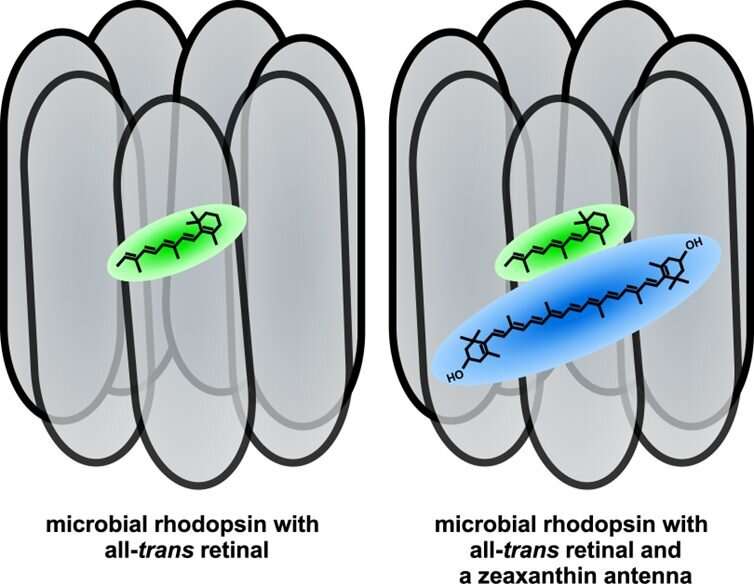
Rhodopsins are the light-driven proton pumps that bacteria employ to produce ATP. Whereas photosynthesis is a process that involves multiple stages and proteins, the rhodopsin performs everything itself. It is not more efficient, but rather it is like the difference between a medieval workshop and a modern factory. The rhodopsins are activated by a molecule called “retinal,” which absorbs light. Specifically, in these proteins retinal absorbs green light. A different molecule, a carotenoid “antenna,” can enable it to also absorb blue light as well, increasing the amount of energy the rhodopsin can produce.
However, these antennae have so far been found only in two rare bacteria species, whereas half the bacteria living in ocean and lake surfaces contains a rhodopsin gene.
To graduate student Ariel Chazan, working under the supervision of Professor Oded Béjà from the Technion Faculty of Biology, this seemed strange. Being able to absorb light in the blue range is advantageous, as blue light penetrates deeper into the water. And carotenoids are widely available in nature. Could it be that a helpful tool would be lying around, and no bacteria would pick it up? Mr. Chazan hypothesized that rather, the antennae used by many bacteria have not yet been discovered. And he set out to find them.

How do you find a molecule without knowing what exactly you’re looking for? Mr. Chazan went fishing. He collected water from Lake Kinneret, and isolated known rhodopsin proton-pumps. Then he used them as bait to fish for potential antennae in the same water. Molecules that got attached to the rhodopsins and increased their energy output under blue light were the ones he was looking for. He found many. Many variants of molecules that scientists had not been familiar with in the context of rhodopsins, and that microbes were apparently using to generate more energy from the light they were exposed to.
It is one thing for something to occur in Lake Kinneret. But if the same thing occurs in oceans all across the world, that’s groundbreaking. Mr. Chazan proceeded therefore to perform the same experiments on ocean water. He was also working to prove something else as well: that the molecules he found were effective rhodopsin-antennae not only in a test tube, but also inside the living cells. All experiments proved positive.
“This is new knowledge about the primary producers on earth—the organisms that produce energy available to living things from inorganic energy sources. Other organisms eat those, and so use the energy that’s already in the system. So, we found out that more energy is entering the food chain than was previously known,” Mr. Chazan said, explaining the importance of his discovery. The scientific community is in agreement that this study has far-reaching implications, and it was recently published in Nature.
The work was performed by an international team, including groups from Japan, Spain and Israel. The “fishing” methodology Mr. Chazan used is an old one, almost an outdated one. “People were a little skeptical when I proposed it,” he said.
“But I like applying existing techniques in ways they weren’t used before. We shouldn’t forget old tools just because there’s something newer and shinier in our toolbox. Going out into the field, seeing what nature gives us, takes more effort than ordering clean industrially produced kits and doing everything in the lab. But those sterile kits are farther away from the nature we wish to study, and things get lost in the transition.”
More information:
Ariel Chazan et al, Phototrophy by antenna-containing rhodopsin pumps in aquatic environments, Nature (2023). DOI: 10.1038/s41586-023-05774-6
Citation:
Light-to-energy conversion in many aquatic microbes more complex than was previously known, researchers discover (2023, March 6)
retrieved 6 March 2023
from https://phys.org/news/2023-03-light-to-energy-conversion-aquatic-microbes-complex.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

