Hydro/dehydrogenation of N‐heterocycles over bifunctional MoNi₄ electrode with water
The catalytic hydrogenation of N-heteroarenes showcases wide and important applications in the fields of synthetic chemistry, drug discovery, materials science, and hydrogen storage. However, it remains a long-standing scientific and technological challenge in breaking the aromaticity of substrates and overcoming catalyst poisoning by either substrates or hydrogenated products. Although different homogeneous systems mainly based on precious metal catalysts have been developed, harsh conditions with extra additives are always required.
Furthermore, homogeneous catalysis not only requires expensive ligands but also has an inherent limitation in recycling, hindering its large-scale application. In contrast, the heterogeneous systems offer a promising alternative since the catalysts can be easily recovered and reused. However, both homogeneous and heterogeneous catalytic hydrogenation methods generally require flammable and explosive gaseous H2 or other expensive/toxic hydrogen sources, causing severe safety risks and environmental concerns.
Furthermore, deuterium labeling plays a vital role in the pharmaceutical industry because of its kinetic isotope effect. N-heterocycles are common structural motifs in alkaloids and bioactive molecules of drugs and agrochemicals. But the reported strategies exhibit low compatibility with the deuterated N-heterocyclic compounds owing to the hard access to suitable deuterium sources. Thus, developing a facile and sustainable hydrogenation (deuteration) strategy of N-heteroarenes over low-cost non-noble metal catalysts with cheap and safe hydrogen (deuterium) sources is highly desirable.
Aqueous electrocatalytic hydrogenation (ECH) is emerging as a promising and sustainable approach for reducing multifunctional chemicals, such as CO2, NO3–, and biomass. Unlike the traditional methods, aqueous ECH uses the electrochemically in situ generated active hydrogen atom (H*) on the electrode surface by proton reduction or water splitting for the synthesis of hydrogenated products. It avoids the use of high pressure and flammable H2 and other expensive and toxic hydrogen donors, demonstrating good economy and sustainability.
In this regard, electrochemical transfer hydrogenation is highly recommended as an appealing strategy for the hydrogenation of organic compounds. Currently, the most studied cases of organic ECH are focused on the reduction of carbonyl and nitro groups. However, those highly conjugated and stable arenes are rarely touched, and still remains a challenging task. Accordingly, it is significant to achieve ECH of N-heteroarenes by exploiting appropriate cathode material for efficiently generating H* and adjusting the adsorption and desorption process of organic molecules on the electrode surface.
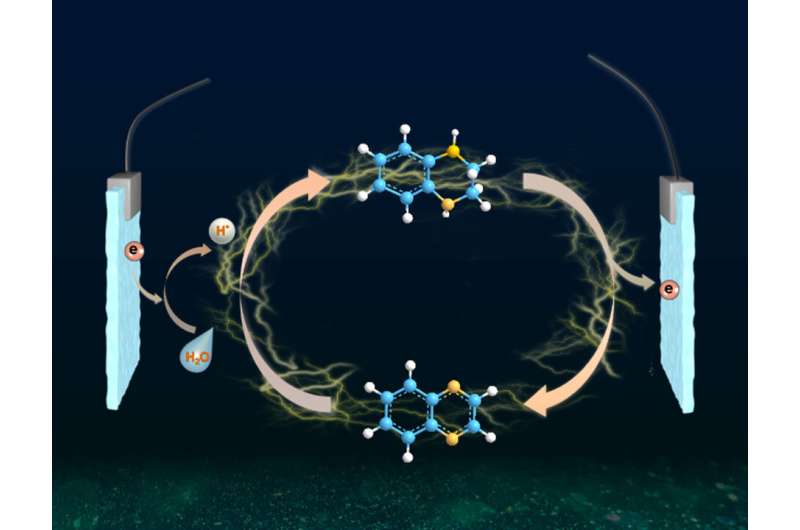
Recently, a research team led by Prof. Bin Zhang from Tianjin University, China reported a room-temperature electrochemical strategy for hydrogenation (deuteration) and reverse dehydrogenation of N-heterocycles over a bifunctional MoNi4 electrode, which includes the hydrogenation of quinoxaline using H2O as the hydrogen source with 80% Faradaic efficiency and the reverse dehydrogenation of hydrogen-rich 1,2,3,4-tetrahydroquinoxaline with both up to 99% yields and selectivity.
The in situ generated active hydrogen atom (H*) might be involved in the hydrogenation of quinoxaline, and a consecutive hydrogen radical coupled electron transfer pathway is proposed. Notably, the MoNi4 alloy exhibits efficient quinoxaline hydrogenation at only 50 mV overpotential owing to its superior water dissociation performance to provide H* in alkaline media. In situ Raman tests indicate that the NiII/NiIII redox can promote the dehydrogenation process, representing a promising anodic alternative to low-value oxygen evolution. Impressively, electrocatalytic deuteration is easily implemented with up to 99% deuterated ratios by using D2O. This method is capable of producing a series of functionalized hydrogenated and deuterated quinoxalines. The results were published in the Chinese Journal of Catalysis.
Hydrogenation regulation of nitrobenzene in electrocatalytic processes realized
Mengyang Li et al, Water-involving transfer hydrogenation and dehydrogenation of N-heterocycles over a bifunctional MoNi4 electrode, Chinese Journal of Catalysis (2021). DOI: 10.1016/S1872-2067(21)63834-2
Provided by
Chinese Academy Sciences
Citation:
Hydro/dehydrogenation of N‐heterocycles over bifunctional MoNi₄ electrode with water (2021, October 22)
retrieved 22 October 2021
from https://phys.org/news/2021-10-hydrodehydrogenation-nheterocycles-bifunctional-moni-electrode.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

