Development of a new method of synthesizing dialkyl ethers using three catalysts that hydroxylate alkenes
Just when we thought alkenes could only react with alcohol to yield ethers in the presence of strong acids, hydroalkoxylation may not be quite what we expected. Organic Chemistry 101 will never be the same again, but the pharmaceutical industry may see the light.
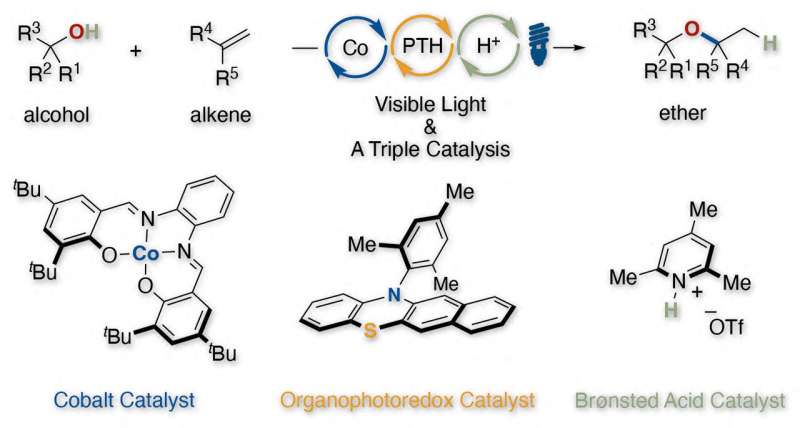
Researchers at Kyoto University have announced the development of a new method of synthesizing dialkyl ethers. Rather than using conventional methods with strong acids—entailing practical challenges such as with acid-sensitive functional groups—the team has devised a protocol using three catalysts that hydroxylate alkenes quickly and cheaply.
This triple catalysis consists of cobalt, organic photoredox, and weak Brønsted acid catalysts.
“Our discovery allows us to synthesize pharmaceutically relevant and highly functional dialkyl ether skeletons in just one rapid step, using relatively inexpensive and accessible raw materials,” explains lead author Hirohisa Ohmiya.
The three catalysts enable the precise control of electrons and protons to convert unactivated alkenes to the corresponding reactive carbocation equivalents in ethers under mild reaction conditions.
As a bonus, this catalysis has demonstrated its ability to freely utilize internal alkene partners other than terminal ones, making secondary and tertiary alkylation of alcohol reactants feasible.
The author adds that they “were also impressed that the valence of cobalt can fluctuate during the triple catalytic cycle to achieve valence-dependent functions.”
“Our three catalysts perform their independent functions in a single flask to create value-added molecules efficiently and economically, possibly accelerating novel drug discoveries.”
The research was published in the Journal of the American Chemical Society.
Light as a tool for the synthesis of complex molecules
Masanari Nakagawa et al, A Triple Photoredox/Cobalt/Brønsted Acid Catalysis Enabling Markovnikov Hydroalkoxylation of Unactivated Alkenes, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c00527
Citation:
Development of a new method of synthesizing dialkyl ethers using three catalysts that hydroxylate alkenes (2022, August 4)
retrieved 4 August 2022
from https://phys.org/news/2022-08-method-dialkyl-ethers-catalysts-hydroxylate.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

