Chemists use light energy to produce small molecular rings
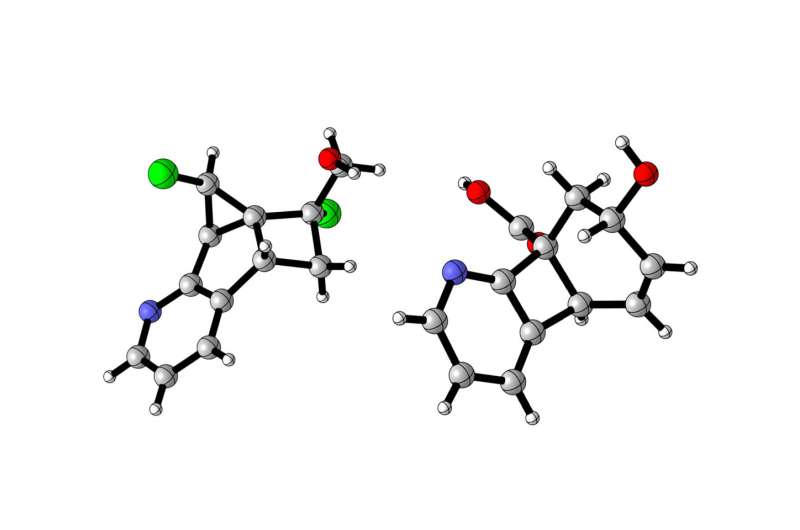
In the search for new active agents in medicine, molecules whose atoms are linked in rings are becoming increasingly important. Such ring systems have particularly suitable properties for producing such active agents and they are driving the development of innovative treatments for malignant tumours, as well as for neurodegenerative and infectious diseases. A team of chemists headed by Prof. Frank Glorius from the University of Münster has now succeeded in synthesising new and medically significant small molecular rings, which are difficult to produce because they are particularly sensitive. The team’s work has been published in the journal Nature Catalysis.
Among chemists, especially the synthesis of small ring systems from so-called aromatic compounds is considered to be difficult. Also, an especially large amount of energy is needed for the process. A further hurdle is that the energy has to be released selectively to the source materials, but not to the heat-sensitive products. Frank Glorius’ team has now developed a strategy in which visible light, as an inexpensive energy source, activates a photocatalyst which drives the reaction. The photocatalyst absorbs the light and transfers its energy to the source materials. In this way, it enables synthesis to take place which is highly efficient and mild and which has no, or hardly any, undesired side-reactions.
“We see our study as a breakthrough in synthesis chemistry,” says lead author Dr. Jiajia Ma. “It shows that light energy can be used in a targeted way to produce small ring systems. The fact that, by using different reaction partners, we can produce different ring systems provides numerous opportunities for the production of active agents.” For their source materials, the chemists used only easily available, inexpensive raw materials.
Chemists discover new reactivity of strained molecules
Jiajia Ma et al, Facile access to fused 2D/3D rings via intermolecular cascade dearomative [2 + 2] cycloaddition/rearrangement reactions of quinolines with alkenes, Nature Catalysis (2022). DOI: 10.1038/s41929-022-00784-5
Provided by
University of Münste
Citation:
Chemists use light energy to produce small molecular rings (2022, May 26)
retrieved 26 May 2022
from https://phys.org/news/2022-05-chemists-energy-small-molecular.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

