Chemists develop new method for water splitting
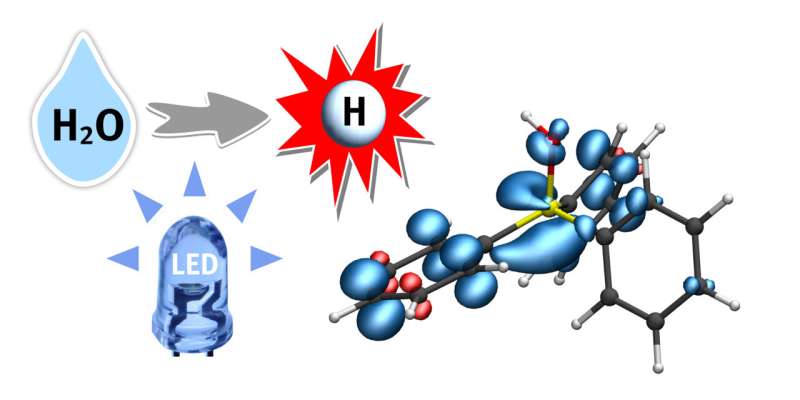
Hydrogen is seen as an energy source of the future—at least, when it is produced in a climate-friendly way. Hydrogen can also be important for the production of active ingredients and other important substances. To produce hydrogen, water (H2O) can be converted into hydrogen gas (H2) by means of a series of chemical processes. However, as water molecules are very stable, splitting them into hydrogen and oxygen presents a big challenge to chemists. For it to succeed at all, the water first has to be activated using a catalyst; then it reacts more easily.
A team of researchers led by Prof. Armido Studer at the Institute of Organic Chemistry at Münster University (Germany) has developed a photocatalytic process in which water, under mild reaction conditions, is activated through triaryl phosphines, and not—as in most other processes—through transition metal complexes.
This strategy, which has now been published in Nature, will open a new door in the highly active field of research relating to radical chemistry, says the team. Radicals are, as a rule, highly reactive intermediates. The team uses a special intermediate—a phosphine-water radical cation—as activated water, from which hydrogen atoms from H2O can be easily split off and transferred to a further substrate. The reaction is driven by light energy.
“Our system,” says Prof. Studer, “offers an ideal platform for investigating unresearched chemical processes which use the hydrogen atom as a reagent in synthesis.”
Dr. Christian Mück-Lichtenfeld, who analyzed the activated water complexes using theoretical methods, says, “The hydrogen-oxygen bond in this intermediate is extraordinarily weak, making it possible to transfer a hydrogen atom to various compounds.”
Dr. Jingjing Zhang, who carried out the experimental work, adds, “The hydrogen atoms of the activated water can be transferred to alkenes and arenes under very mild conditions, in so-called hydrogenation reactions.”
Hydrogenation reactions are enormously important in pharmaceutical research, in the agrochemical industry and in materials sciences.
More information:
Jingjing Zhang et al, Photocatalytic phosphine-mediated water activation for radical hydrogenation, Nature (2023). DOI: 10.1038/s41586-023-06141-1
Citation:
Chemists develop new method for water splitting (2023, June 28)
retrieved 28 June 2023
from https://phys.org/news/2023-06-chemists-method.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

