Carbon nanotube membranes with Pd-Cu modification successfully reduce nitrate levels via electrocatalysis
The adverse effects of excess nitrate in water on human productivity and lives have received increasing attention due to the discharge of industrial wastewater and the overuse of farmland fertilizers. An international team of researchers has conducted an in-depth study of the significant need and challenge of efficient nitrate removal.
Several techniques have been used to eliminate nitrate from water, such as biological denitrification is technologically mature, cost-effective, and widely used. However, biological processes are often sluggish and easily affected by variable environmental conditions, such as temperature and the availability of carbon supplies. The researchers suggested that electrocatalytic technology is a promising method for nitrate removal because no chemicals are needed, no sludge is produced, and implementation of the methodology is convenient.
Most of the electrodes currently used for electrocatalytic nitrate reduction are conventional plate cathodes implemented in parallel plate reactors where mass transfer is strongly limited. Consequently, the overall electrocatalytic nitrate reduction rate decreases. Novel reactive electrochemical membranes (REMs) for nitrate reduction had previously been reported, but the synthetic processes for these proposed materials are complicated and the high cost of preparation have hindered the practical application of REMs.
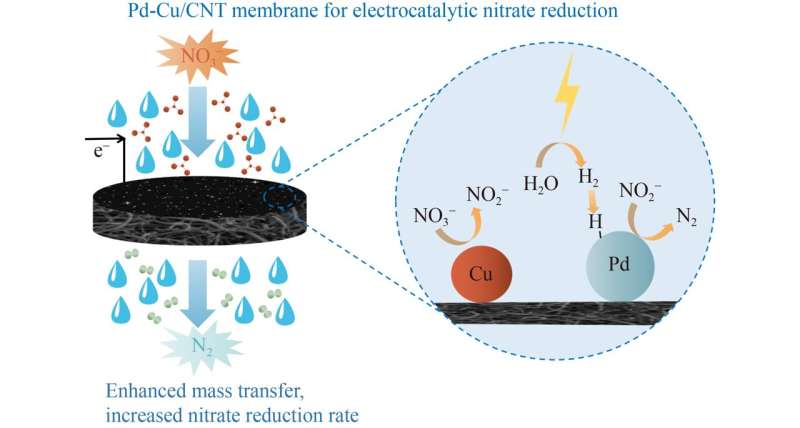
To address these barriers, researchers from Minzu University of China and Wuhan University prepared a Pd-Cu modified carbon nanotube membrane was fabricated with an electrodeposition method and used to reduce nitrate in a flowthrough electrochemical reactor.
Their study reveals that the membrane obtained with a Pd : Cu ratio of 1:1 exhibited a relatively high nitrate removal efficiency and N2 selectivity. This study entitled “Electrocatalytic reduction of nitrate using Pd-Cu modified carbon nanotube membranes” is published online in Frontiers of Environmental Science & Engineering.
In this study, the research team fabricated a novel Pd-Cu modified carbon nanotube membrane cathode on polyvinylidene fluoride (PVDF) substrate to overcome the mass transfer problem during nitrate reduction.
Initially, to determine the optimal potential for electrocatalytic nitrate reduction in flow-through mode, nitrate reduction was conducted with Pd-Cu modified CNT membranes with different potentials ranging from −2.0 to −0.4 V. The team noted that the optimal potential and duration for codeposition of Pd and Cu were −0.7 V and 5 min, respectively, according to linear scan voltammetry results for CNT membranes in different solutions.
The second question the team examined was the effect of the Pd-Cu molar ratio and electrode potential on nitrate removal. The team noted that the membrane obtained with a Pd : Cu ratio of 1:1 exhibited a relatively high nitrate removal efficiency and N2 selectivity.
Nitrate was almost completely reduced (~99 %) by the membrane at potentials lower than −1.2 V. However, −0.8 V was the optimal potential for nitrate reduction in terms of both nitrate removal efficiency and product selectivity. The nitrate removal efficiency was 56.2 %, and the N2 selectivity was 23.8 % for the Pd : Cu=1:1 membrane operated at −0.8 V.
The third question the team investigated was the effect of solution conditions. They noted that nitrate removal was enhanced under acidic conditions, while N2 selectivity was decreased. The concentrations of Cl− ions and dissolved oxygen showed little effect on nitrate reduction.
In addition, to illustrate the effect of flow-through operation on mass transfer, the team evaluated the effect of membrane flux on nitrate reduction and calculated the mass transfer rate constants at different membrane fluxes.
They note that the mass transfer rate constant was greatly improved by 6.6 times from 1.14 × 10−3 m/h at a membrane flux of 1 L/(m2·h) to 8.71 × 10−3 m/h at a membrane flux of 15 L/(m2·h), which resulted in a significant increase in the nitrate removal rate from 13.6 to 133.5 mg/(m2·h).
Pd-Cu modified CNT membranes were successfully prepared via the electrodeposition method, and a flow-through electrocatalytic system was constructed to reduce nitrate in this study. This technology has broad application prospects for effective nitrate reduction and is worthy of further modification in practice.
More information:
Zhijun Liu et al, Electrocatalytic reduction of nitrate using Pd-Cu modified carbon nanotube membranes, Frontiers of Environmental Science & Engineering (2022). DOI: 10.1007/s11783-023-1640-1
Provided by
Higher Education Press
Citation:
Carbon nanotube membranes with Pd-Cu modification successfully reduce nitrate levels via electrocatalysis (2023, May 12)
retrieved 12 May 2023
from https://phys.org/news/2023-05-carbon-nanotube-membranes-pd-cu-modification.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

