Breaking the barrier: Low-temp ammonia synthesis with iron catalysts and barium hydride
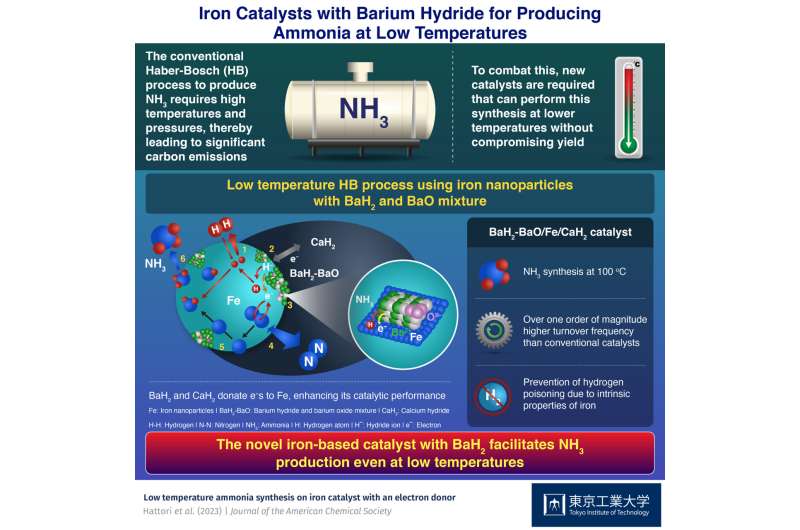
The Haber-Bosch (HB) process is one of the most important industrial chemical reactions. It combines nitrogen and hydrogen gases in the presence of an iron-based catalyst at high temperatures and pressures to produce ammonia fertilizer which helps provide food for over five billion people.
Over the decades, researchers have tried to bring down the reaction temperature of the HB process to increase the ammonia yield while reducing energy consumption. To this end, they have recently developed new catalysts based on other transition metals, such as ruthenium, cobalt, and nickel, which exhibit much higher catalytic activity than iron.
However, these catalysts preferentially adsorb hydrogen atoms onto their surface at low temperatures of 100–150oC, which reduces nitrogen adsorption and thus hampers ammonia production. This phenomenon, known as hydrogen poisoning, poses an obstacle to the low-temperature HB process.
In this light, researchers led by Professor Michikazu Hara of the Laboratory for Materials and Structures at Tokyo Institute of Technology (Tokyo Tech), have refocused on iron-based catalysts and modified them to produce ammonia at 100oC. Their work is all set to be published in the Journal of the American Chemical Society.
Prof. Hara explains the motivation behind the research. “Hydrogen poisoning is not strong for iron-based catalysts. Therefore, they may be used for the low-temperature HB process but only when combined with an appropriate promoter that increases their catalytic activity.” In this work, the researchers prepared metallic iron (Fe) nanoparticles on calcium hydride (CaH2) particles, with a mixture of barium oxide (BaO) and barium hydride (BaH2) deposited on them.
A set of experiments revealed that the iron nanoparticles interact strongly with the hydride ions of both hydrides. As a result, hydrogen atoms move from the hydrides to the nanoparticles and get desorbed as hydrogen gas, leaving behind electrons. The hydrides donate these electrons to the iron nanoparticles. It facilitates the breaking of nitrogen gas into atoms, resulting in enhanced catalytic activity for ammonia production even at low temperatures.
The BaH2–BaO/Fe/CaH2 catalyst exhibited a turnover frequency of 0.23 s-1 at 100oC and 12.3 s-1 at 300oC under a moderate pressure of 0.9 MPa. These values, orders of magnitude higher than those for catalysts based on other transition metals, result from the ability of iron to prevent hydrogen poisoning by desorbing the adsorbed hydrogen atoms as hydrogen gas at low temperatures.
Discussing the future potential of their work, Prof. Hara observes, “The BaH2–BaO/Fe/CaH2 catalyst facilitates low-temperature HB process, which consumes less energy. As such, it can reduce the use of fossil fuels and potentially lower global carbon emissions. In addition, iron is abundant and inexpensive, which makes the HB process more sustainable.”
More information:
Michikazu Hara et al, Low temperature ammonia synthesis on iron catalyst with an electron donor, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.2c13015
Citation:
Breaking the barrier: Low-temp ammonia synthesis with iron catalysts and barium hydride (2023, March 30)
retrieved 30 March 2023
from https://phys.org/news/2023-03-barrier-low-temp-ammonia-synthesis-iron.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

