BESSY II: Surface analysis of catalyst particles in aqueous solutions
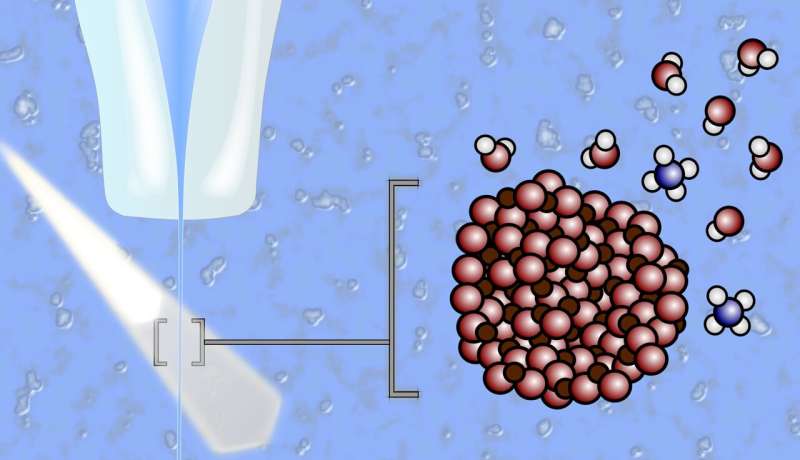
Green hydrogen can be produced directly in a photoelectrochemical cell, splitting water with solar energy. However, this requires the development of super-efficient photoelectrodes that need to combine many talents at the same time: They must be excellent at converting sunlight into electricity, remain stable in acidic or basic water, act as catalysts to promote the splitting of water into hydrogen and oxygen, and be cheap, abundant and non-toxic. The large material class of metal oxides comes into question.
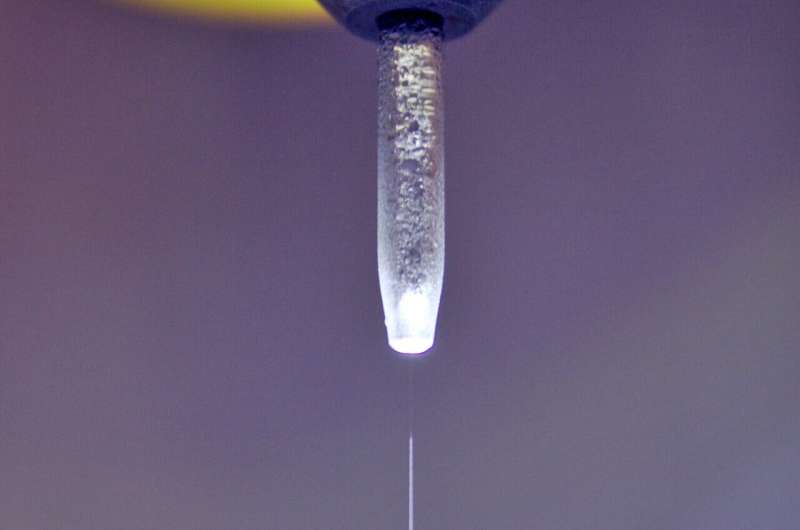
However, it is difficult to find out what really happens at the interfaces between the solid metal oxide electrodes and the aqueous electrolyte. This is because standard X-ray analysis does not work to investigate processes on samples in liquid environments. One of the few suitable methods are experiments with a liquid jet: an extremely fine jet of liquid in which nanoparticles of metal oxide are suspended.
This jet shoots through the X-ray light of BESSY II, and the interference of the evaporated molecules with the measurement data is negligible (more in the foreword to the special issue).
Dr. Robert Seidel is an expert on this liquid jet method, which is the subject of a special issue of Accounts of Chemical Research. He was invited to be the guest-editor of the issue and to report also on new experiments at BESSY II that he conducted with Dr. Hebatallah Ali and Dr. Bernd Winter from the Fritz Haber Institute.
They investigated two important model systems for photoelectrodes: Nanoparticles of iron oxide (hematite, α-Fe2O3, and anatase (titanium oxide or TiO2) in aqueous electrolytes with different pH values. Hematite and anatase in suspension are photocatalytic model systems. They are ideal for studying the solid/electrolyte interface at the molecular level and for exploring the chemical reactions at electrode-electrolyte interfaces.
“We used resonant photoelectron spectroscopy (PES) to identify the characteristic fingerprints of different reactions. This allowed us to reconstruct which reaction products are formed under different conditions, particularly as a function of pH.” The key question: How do the water molecules react with or on the nanoparticle surfaces?
In fact, how acidic or how basic an electrolyte is makes a big difference, Seidel noted. “At low pH, the water molecules on the surface of hematite tend to split. This is not the case with anatase, where water molecules are adsorbed on the surface of the TiO2 nanoparticles,” says Seidel. A basic pH value is required for water molecules to break down on the anatase nanoparticles. “Such insights into surface interactions with water molecules are only possible with this liquid-jet method,” says Seidel.
The spectra also revealed ultra-fast electron transitions between the metal oxide and the (split) water molecules on the surface. The results provide insights into the first steps of water dissociation and help to clarify the mechanisms of light-induced water splitting on metal oxide surfaces.
The special issue ‘Applications of Liquid Microjets in Chemistry’:
“Pour a glass of water, and bring it right up to your nose, one centimeter away. What don’t you see? About 3 million molecules intervene along a line between the tip of your nose and the surface of the water. Imagine an X-ray photon or charged or neutral particle trying to reach or escape the surface but first colliding with some of these interloping molecules in ways that scramble information about their interactions with interfacial and deeper water molecules.”
What is so vividly described in the foreword to this special issue has long been a major problem. It was not until 1988 that the liquid jet method, introduced by Manfred Faubel, Stephan Schlemmer and Jan Peter Toennies, made it possible to study water surfaces without these disturbances. The microjet is a fast-flowing stream of liquid so narrow that it produces only an extremely dilute vapor cloud. Photons and particles can reach and leave the surface of the jet without colliding with the vapor molecules.
More information:
Hebatallah Ali et al, The Metal-Oxide Nanoparticle–Aqueous Solution Interface Studied by Liquid-Microjet Photoemission, Accounts of Chemical Research (2023). DOI: 10.1021/acs.accounts.2c00789
Citation:
BESSY II: Surface analysis of catalyst particles in aqueous solutions (2023, July 21)
retrieved 21 July 2023
from https://phys.org/news/2023-07-bessy-ii-surface-analysis-catalyst.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

