Ahead of ruling that could disrupt access to medication abortion, providers line up alternatives
Washington — Abortion providers nationwide are preparing for a ruling from a federal judge in Texas that could disrupt access to a key drug used to facilitate medication abortion, and have been making plans to ensure there is an alternative available for patients if needed.
The forthcoming decision from U.S. District Judge Matthew Kacsmaryk is in response to a request from anti-abortion medical associations to order the Food and Drug Administration (FDA) to withdraw its approval of the drug mifepristone.
Kacsmaryk has scheduled a hearing on Wednesday for the physicians groups and Biden administration to press their arguments about the legality of mifepristone’s approval, according to The Washington Post. The Amarillo-based judge could issue a decision after the hearing at any time.
The FDA first approved mifepristone in 2000. In their suit, the associations argue the agency erred in determining its safety and effectiveness, and claim it exceeded its regulatory authority by approving the abortion pill.
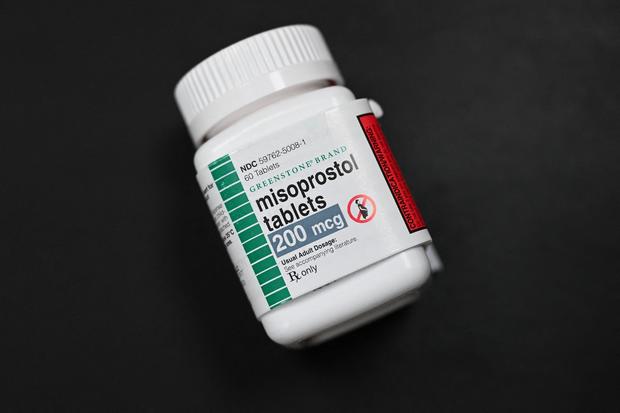
Mifepristone is the first of a two-drug regimen that is used to terminate a pregnancy through 10 weeks gestation. In anticipation of an injunction from Kacsmaryk, abortion providers are preparing to offer patients the option to use solely misoprostol, the second medication, which is approved by the FDA for the prevention and treatment of gastric ulcers.
“They work very well together,” said Melissa Grant, chief operations officer of carafem, an organization that provides online and in-person abortion services, of mifepristone and misoprostol. Misoprostol can be used alone when mifepristone is not available, she said, though “it is a process, and people have to be willing to invest the time it takes to complete the process.”
Carafem has been offering medication abortions using only misoprostol since 2020, when the COVID-19 pandemic heightened the need for easier accessibility to the abortion pill. At the time, Grant said more than 80% of carafem’s clients elected to use misoprostol only when given the choice.
“It became an important option during the pandemic” and allowed carafem the opportunity to provide better access to certain populations, she said.
The proportion of patients opting to take only misoprostol to terminate their pregnancies fell to between 10% and 15% after the FDA relaxed in-person dispensing requirements in 2021, allowing providers to use telemedicine to prescribe mifepristone and patients to obtain it by mail.
ROBYN BECK/AFP via Getty Images
Grant predicted that if providers are restricted from providing mifepristone as a result of a judicial order, more women seeking medication abortions may opt for the misoprostol-only option.
Under the two-drug regimen, a single mifepristone pill is taken first to block progesterone, the hormone necessary for a pregnancy to develop. Between 24 and 48 hours later, the patient will then take misoprostol to bring on contractions, a dose that consists of four pills that can be dissolved in the mouth or taken vaginally.
The two-drug sequence is between 95% and 99% effective in ending a pregnancy, and medication abortions made up more than 50% of all abortions in the U.S. in 2020, according to the Centers for Disease Control and Prevention. A study from the Guttmacher Institute, a pro-abortion rights research organization, found that 98% of medication abortions in the U.S. used the mifepristone and misoprostol combination.
If Kacsmaryk orders the FDA to rescind its approval of mifepristone, the process could take months, and it would not mark the end of medication abortion in the U.S. Instead, patients could use misoprostol alone, which studies show is between 80% to 100% effective and is recommended by the World Health Organization as a safe and effective alternative. To use misoprostol only, a total of 12 pills are taken in three doses every three hours.
“We know that there’s just a slight difference in efficacy, but it’s still safe and effective,” said Dr. Julie Amaon, medical director of Just The Pill, referring to using misoprostol on its own. “The biggest thing that we’re preparing for is the mass confusion that happens after most of these decisions come down.”
As with the recent changes in the legal landscape since the Supreme Court ended the constitutional right to abortion in June 2022, Amaon said her organization is working to prevent interruptions for its patients.
“We are in the wild, wild west,” she said. “Everyone is just unsure about what this ruling could mean.”
With a decision from Kacsmaryk looming, the National Abortion Federation, the professional organization of abortion providers, recently amended its policy guidelines for abortion care to recommend that “where mifepristone is either not legally available or inaccessible, misoprostol-alone regimens or other evidence-based regimens may be offered.”
“We’re trying to make sure people understand what’s happening and that medication abortion is still safe and available,” Melissa Fowler, the federation’s chief program officer, told CBS News.
For abortion providers, ensuring that patients are educated about the impacts of a decision involving abortion pills and the options available to them is crucial. Carafem’s staff has been briefed on how to talk about and teach the misoprostol-only protocol to ensure it is most effective, and the organization has volunteered to help other groups to make sure they understand how the one-drug regimen works.
“Every time there’s a change, it can send vulnerable people into a tailspin, so we think it’s important to share as much as we can about what alternative options may be,” Grant said.
Just The Pill and Hey Jane, a virtual abortion clinic, are also prepared to shift to a misoprostol-regimen.
Suppliers of misoprostol, such as online pharmacies like Honeybee Health, have assured providers that they are not concerned about meeting a possible increase in demand.
“The judicial system does not regulate drugs, so we need to see how much authority [Kacsmaryk] really has to overrule FDA approval,” Kiki Freedman, co-founder and CEO of Hey Jane, said. “Hopefully it doesn’t come to that and we will do everything we can within legal bounds, but we will certainly do everything we can to continue providing care to our patients.”
Clinics are already grappling with an influx of patients seeking abortions sparked by the Supreme Court’s reversal of Roe v. Wade, which cleared the way for limits or outright bans to take effect in more than a dozen states.
“We’re already overstressed at many of the clinical buildings that provide abortion care because they’ve started to serve people from banned states that are traveling,” Grant said.
Further disruptions to access to the abortion pill will only further stress brick-and-mortar health centers that provide surgical abortions and state health care systems, a consequence the FDA has warned of.
“Taking away patients’ option of medication abortion would lead to overcrowding and delays at clinics that provide surgical abortion, including not only dedicated abortion clinics but also general practitioners,” the agency told Kacsmaryk in a filing. “This would lead to delays for an array of healthcare services as providers and resources are unnecessarily diverted to surgical abortions.”
Abortion rights groups are also concerned that the legal challenge to the FDA’s approval of mifepristone could have ramifications that reach beyond the borders of Texas, where the suit was brought, and lead to lawsuits targeting other drugs.
“If he orders the FDA to withdraw its approval, it would affect every state. It would affect the places where abortion is protected,” Fowler said. “This case is bigger than abortion and it has consequences that could affect and undermine the FDA approval process. We don’t want to set a precedent where a small group of people could interfere with or overturn the FDA approval of medications just because they don’t like the medication. We need to be trusting the science.”
For all the latest Automobiles News Click Here
For the latest news and updates, follow us on Google News.