A way to conduct Birch reductions that does not involve ammonia
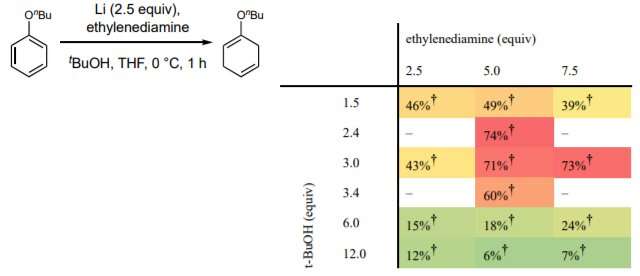
A team of chemists at the University of Pittsburgh has developed a new way to conduct Birch reductions that does not require the use of ammonia, thus avoiding a dangerous procedure. In their paper published in the journal Science, the group describes the new method and the ways it can be used.
In chemistry, Birch reductions are organic reactions that convert arenes (aromatic hydrocarbons) to cyclohexadienes (six-carbon alicyclic hydrocarbons). They are typically used to build other complex molecules. The traditional method involves dissolving alkali metals in liquid ammonia—doing so produces the solvated electrons that drive the reaction. Importantly, such chemicals are hazardous, as is combining them. Chemists have been looking for a better alternative for many years. To date, most such efforts have resulted in processes deemed too hard to control, too expensive, or that require cryogenic conditions. The researchers note that some have suggested the Benkeser reduction can be used as a suitable replacement, but not for producing 1,4-cyclohexadienes. In this new effort, the team has found a new way to conduct Birch reductions without using ammonia. The process does not suffer from the drawbacks of the other modified procedures.
In their new method, the researchers replaced ammonia with tetrahydrofuran (a clear liquid that is often used as a plastics solvent) and make use of both lithium and an inexpensive ethylenediamine reagent to carry out the reaction. They tested their new approach by converting samples of benzene to cyclohexadienes. In addition to removing the dangerous elements from Birch reductions, the new ingredients allow the method to be conducted at room temperature. The traditional method involved cooling the materials to approximately -33 degrees Celsius to prevent the ammonia from evaporating. The new method is also easily scaled up, is inexpensive and can be conducted in virtually any laboratory setting. The researchers claim that it can also be used to reduce instances of either electron-poor or electron-rich substrates and is compatible with organocuprate chemistry.
Chemists discover new way to harness energy from ammonia
James Burrows et al, Scalable Birch reduction with lithium and ethylenediamine in tetrahydrofuran, Science (2021). DOI: 10.1126/science.abk3099
© 2021 Science X Network
Citation:
A way to conduct Birch reductions that does not involve ammonia (2021, November 16)
retrieved 16 November 2021
from https://phys.org/news/2021-11-birch-reductions-involve-ammonia.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

