A chemoproteomic platform monitors Fe-S cluster occupancy across the E. coli proteome
Boston College chemists have developed a strategy to monitor the presence or absence of iron-sulfur clusters, which are essential to the function of diverse proteins, the team reported recently in the journal Nature Chemical Biology.
The iron-sulfur (Fe-S) proteomic platform the team developed allowed them to gain a greater insight into the dynamics of iron-sulfur cluster delivery and binding, according to Professor of Chemistry Eranthie Weerapana and Senior Research Associate Daniel Bak, co-authors of the study.
Iron-sulfur clusters are found in proteins that are essential to human health, such as DNA repair enzymes, and important in the energy sciences for biofuel cells and cleaner industrial processes.
However, the study of iron-sulfur clusters has always been challenging because of the instability and dynamics of the Fe-S cluster-protein complex, according to Bak.
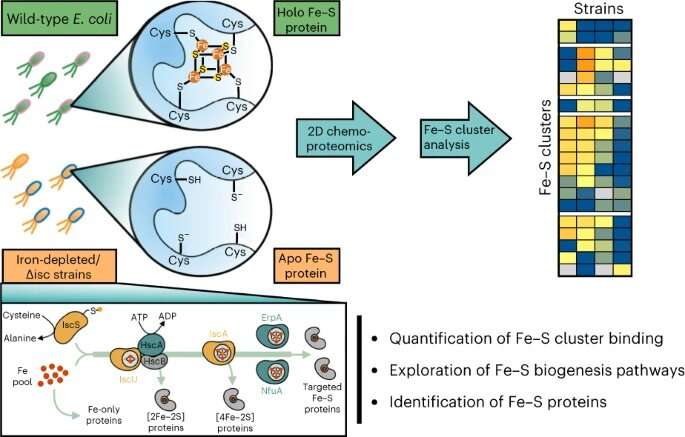
“We set out to develop a technology that would allow us to globally assess the dynamics of Fe-S cluster binding in an entire proteome without the need for laborious protein purification, radioisotopes of iron or sulfur, or previous knowledge of cluster binding sites,” Bak said.
Many proteins contain metal cofactors, such as iron-sulfur clusters, he explained. These inorganic cofactors allow for unique protein functions, such as one-electron redox chemistry, that are not available to the 20 canonical protein amino acids.
The team performed the study in the bacteria E. coli, which is a model organism with a well-characterized iron-sulfur proteome. To measure the binding of Fe-S clusters to protein targets, the team used advanced mass spectrometry-based proteomic strategies coupled with chemical probes that can differentiate between the cluster-bound and -unbound forms of a protein, said Weerapana.
The team observed differential sensitivity of iron-sulfur clusters to both iron-limitation and impairment of the pathways E. coli use to synthesize Fe-S clusters, suggesting a prioritization of iron-sulfur cluster delivery in E. coli, the team reports. Knowledge of iron-sulfur cluster prioritization can provide insight into how different microbes can assimilate and thrive within environmental niches where iron is limited.
The researchers were also able to differentiate the roles of the various iron-sulfur biosynthetic enzymes, some of which are essential for delivery of almost all Fe-S clusters, while others play more specific roles in the delivery of clusters to a specific subset of Fe-S proteins.
“Mining the datasets which we generated, we could compile a list of potentially novel Fe-S proteins, two of which were validated as iron-sulfur cluster binding proteins,” said Weerapana. “This opens the possibility of using proteomics to identify novel Fe-S cluster biology not only in E. coli, but other organisms as well.”
The researchers said the next step is to translate this work to other organisms and model systems, including cell culture models of Fe-S-dependent human diseases such as Friedrich’s Ataxia. The new platform offers a powerful tool to characterize mechanisms of Fe-S cluster synthesis and delivery, as well as identify new iron-sulfur-dependent cellular pathways.
More information:
Daniel W. Bak et al, Monitoring Fe–S cluster occupancy across the E. coli proteome using chemoproteomics, Nature Chemical Biology (2023). DOI: 10.1038/s41589-022-01227-9
Citation:
A chemoproteomic platform monitors Fe-S cluster occupancy across the E. coli proteome (2023, February 8)
retrieved 8 February 2023
from https://phys.org/news/2023-02-chemoproteomic-platform-fe-s-cluster-occupancy.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

