Investigating the effects of amide-to-ester substitutions on membrane permeability of cyclic peptides
Cyclic peptides often exhibit low membrane permeability that can be significantly improved via amide-to-ester substitutions, as demonstrated by researchers from Tokyo Institute of Technology (Tokyo Tech). The utilization of substitutions shown in a new study can be used to develop cyclic peptides with high membrane permeability and oral bioavailability for clinical and therapeutic applications.
Interest in cyclic peptides, a class of organic molecules, has reached a new high recently. Their ability as inhibitors has made them potential candidates for clinical and therapeutic applications. Unlike their linear counterparts, peptides with macrocyclization have better resistance to proteolysis—the disintegration of peptides into amino acids in the presence of enzymes—which makes them stable in the bloodstream, a desired property for any material being used for therapeutic purposes.
However, the membrane permeability—the ability of a molecule to pass through cell membranes in our body—of cyclic peptides is quite low in general. This downside not only makes formulation of orally bioavailable peptides difficult, but also severely affects their ability to target intracellular protein interactions.
On the other hand, some natural cyclic peptides show great membrane permeability and oral bioavailability. These naturally occurring membrane-permeable cyclic peptides often have N-methylamide bonds, ester bonds or both on their backbone, instead of amide bonds that are the most common for amino acids in proteins and peptides. While studies have explored the effects of N-methylation on cyclic peptides, the impact of amide-to-ester substitution has remained unexplored.
That was until a multinational and interdisciplinary group of researchers from Tokyo Tech, The University of Tokyo, and University of California, Santa Cruz, came together to discover the unknown. In their recent breakthrough published in Nature Communications, the team reported a direct evaluation of amide-to-ester substitutions and their effect on the membrane permeability of cyclic peptides.
Prof. Yutaka Akiyama from Tokyo Tech, a corresponding author of this paper, explains, “We know that naturally occurring depsipeptides, which are peptides where one or more of the amide bonds is replaced with an ester bond, show high membrane permeability. This is why our team decided to see if amide-to-ester substitutions can improve membrane permeability. In the team, Tokyo Tech scientists employed a newly developed method on enhanced sampling molecular dynamics (MD) simulations to unravel the mechanism behind this.”
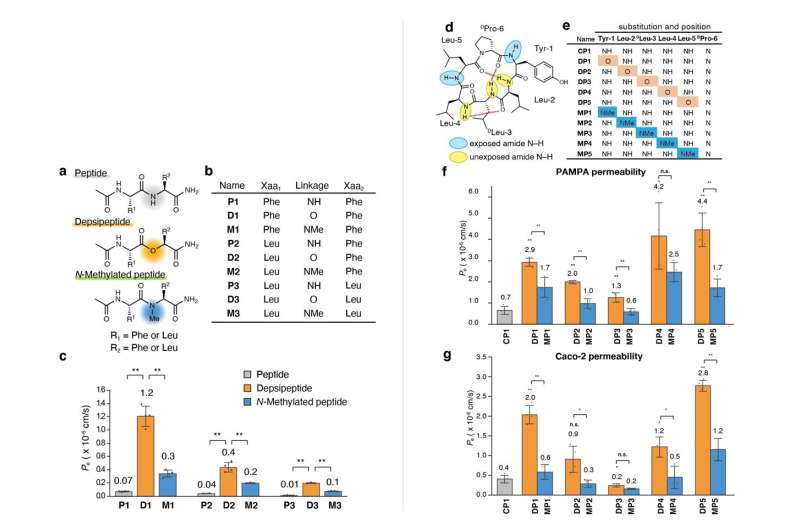
The team first synthesized a series of model dipeptides and their derivatives containing amide-to-ester substitutions. They then compared the membrane permeability of these peptides using wet lab techniques and found that the substituted dipeptides had significantly higher membrane permeability.
To better understand the scope of these substitutions, the team tried amide-to-ester replacements on larger cyclic hexapeptides. The findings indicated that amide-to-ester substitution enhanced the membrane permeability of cyclic hexapeptides much more than the conventional N-methylation process.
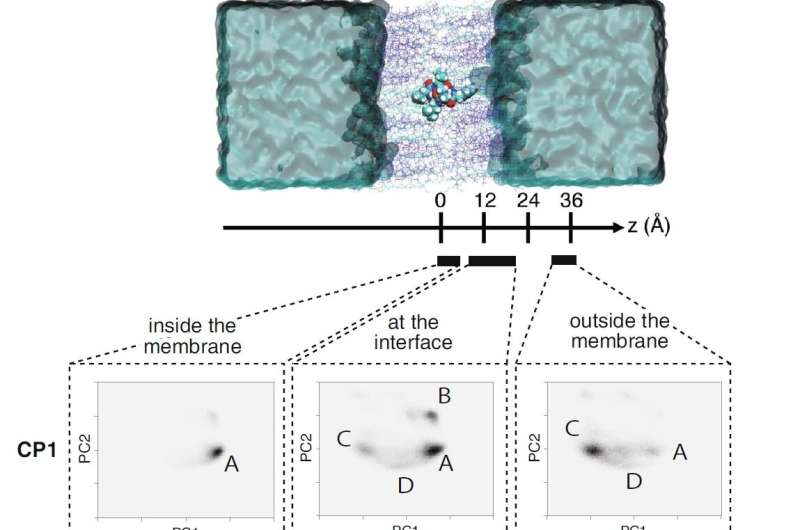
The team also carried out enhanced sampling MD simulations on three cyclic hexapeptides (CP1, DP1, and MP1) using the TSUBAME3.0 supercomputer at Tokyo Tech. The large-scale simulations revealed that the substituted peptides dynamically transition between their open and closed conformations in aqueous solution and at the membrane interface. They also revealed that the amine-to-ester substitution led to a corresponding reduction in the free energy barrier for the substituted peptides, thereby leading to their enhanced membrane permeability.
The insights provided by this study could help expand the utility of cyclic peptides by providing a simple solution to the low permeability problem. “We envision that this amide-to-ester substitution strategy will be utilized for the development of peptides with better oral bioavailability, ability to target intracellular biomolecules, or both,” concludes Prof. Akiyama.
More information:
Nature Communications (2023). DOI: 10.1038/s41467-023-36978-z
Citation:
Investigating the effects of amide-to-ester substitutions on membrane permeability of cyclic peptides (2023, March 17)
retrieved 17 March 2023
from https://phys.org/news/2023-03-effects-amide-to-ester-substitutions-membrane-permeability.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

