Scientists show reduced heavy metal toxicity in goldfish using hard water
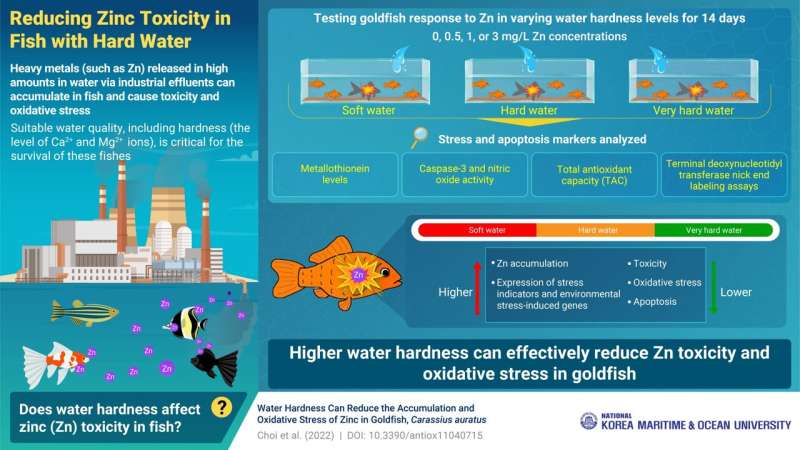
When industrial effluents containing high levels of heavy metals are discharged into fish bodies, they pose a serious threat to aquatic ecosystems. One such heavy metal, zinc, is required by organisms in miniscule amounts, but if it accumulates in higher concentrations, zinc can trigger oxidative stress in the fish body. This causes metabolic, physiological, and cellular damage, including protein degeneration and eventually, cell death. Moreover, humans who consume this fish are also exposed to a high level of zinc, resulting in similarly dangerous consequences for the human body.
While the ideal solution for this problem would be to control the release of effluents into water bodies, there is another way. Maintaining ideal water quality parameters, like hardness (i.e., the level of certain positively charged ions—like calcium and magnesium ions—in water), can inhibit the absorption of heavy metals in fish, and by extension, reduce our exposure to these contaminants.
As evidenced in a new study published in Antioxidants, scientists from the Korea Maritime and Ocean University (KMOU) describe how zinc absorption and toxic stress in goldfish changes at different concentrations of zinc and water hardness. “Compared to seawater, freshwater has a lower concentration of ions, which increases its risk of heavy metal contamination. Therefore, we wanted to explore the possibility of reducing this toxicity in fish by regulating water hardness,” explains Dr. Cheol Young Choi—Professor at KMOU and lead scientist for this study.
Dr. Choi’s team assessed key antioxidant markers and total antioxidant capacity, which indicate the degree of oxidative stress in the body. The team also conducted terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays to determine the severity of apoptosis in the liver, where the antioxidant response is most acute. They studied the oxidative stress progression in goldfish exposed to water having increasing zinc concentrations (plus a control group without any zinc exposure) over a period of 14 days. The team also adjusted the hardness of this water with calcium carbonate.
The results showed a significant difference in zinc accumulation and toxicity in fish who had been exposed to different zinc concentrations and water hardness levels. The fish exposed to the highest concentration of zinc showed the most accumulation and oxidative stress over 14 days. However, for the same concentrations and time, zinc accumulation and stress markers were significantly lower among the fish who were kept in hard, or very hard water. Similarly, the TUNEL assays showed more cellular degeneration and death in zinc-exposed fishes, which reduced with increasing water hardness.
This study demonstrates the effectiveness of higher water hardness in reducing the oxidative stress and bioaccumulation of zinc in fishes. “Bioaccumulated heavy metals are hazardous—not only to fish, but also to humans who later consume these fish. Our study proposes an eco-friendly solution to this problem. Hopefully this will pave the way for further research exploring the contribution of other water quality parameters, like temperature and pH, in reducing heavy metal accumulation in aquatic organisms,” says Dr. Choi.
Cheol Young Choi et al, Water Hardness Can Reduce the Accumulation and Oxidative Stress of Zinc in Goldfish, Carassius auratus, Antioxidants (2022). DOI: 10.3390/antiox11040715
Provided by
National Korea Maritime and Ocean University
Citation:
Scientists show reduced heavy metal toxicity in goldfish using hard water (2022, May 10)
retrieved 11 May 2022
from https://phys.org/news/2022-05-scientists-heavy-metal-toxicity-goldfish.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

