Positive feedback loop drives transition of Toxoplasma gondii to chronic stage
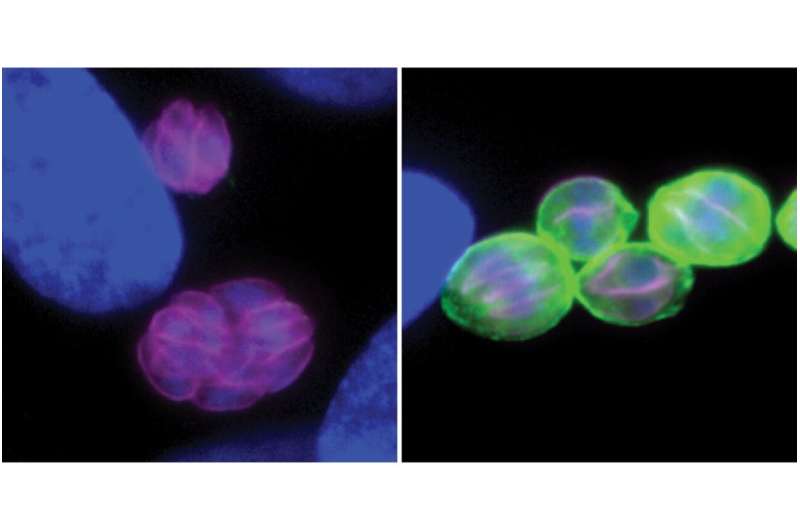
Toxoplasma gondii—otherwise known as Toxo—is a parasite that infects one fourth of the world’s human population. While Toxo remains alive within most of its hosts, the active acute forms of the parasite are quickly suppressed by the immune system and transform into slow-growing chronic cysts.
The symptoms of acute toxoplasmosis—the disease caused by Toxo—can be devastating, especially for immunocompromised and pregnant people. During the AIDS epidemic, many people who carried Toxo developed encephalitis, as the dormant cysts reactivated in brain and muscle tissue. The active infection can also spread within a developing fetus, unchecked by the immune system, which is why people are advised to not change kitty litter while pregnant.
A new paper from Whitehead Institute Member and associate professor of biology at MIT Sebastian Lourido, published online on April 20, 2023, in Nature Microbiology, helps explain how the parasites differentiate, or transition, between acute and chronic phases. The research, led by Lourido lab postdoc Haley Licon, defines a new regulatory circuit that drives differentiation.
Lourido and colleagues had previously found that a Toxo protein called BFD1 acts as the master regulator of chronic differentiation. That is, without BFD1, chronic infection does not occur. Also, expressing BFD1 is enough to cause parasites to transition into their chronic forms. In this publication, Licon found that another protein called BFD2 regulates BFD1 and helps Toxo commit to chronic differentiation.
More than 1,000 genes change expression during chronic differentiation, but only about half of them appear to be directly controlled by BFD1. Licon started out trying to understand how BFD1 was causing parasites to become chronic.
“BFD1 seems to be a positive regulator of gene expression, meaning that when it gets turned on, it activates a bunch of other chronic-stage genes,” she explained. “But we also know it turns on other proteins that may influence expression of genes needed for chronic cyst formation, which would suggest there is some sort of hierarchy in the regulation.” If one were to think of this hierarchy as a phone tree with hundreds of callers, BFD1 would be at the top of this tree.
In order to better understand the hierarchy of genes controlling chronic infection, Licon looked at a set of five genes directly controlled by BFD1 that she thought could be involved. She regulated the expression of each gene in Toxo cells to test its effect on hierarchy of differentiation. Out of the five candidates, one protein behaved similarly to BFD1, and its loss blocked chronic differentiation from happening. The lab named this protein BFD2.
Licon confirmed that in parasites where BFD2 was completely removed, replication progressed normally during the acute phase, but not in the chronic phase. Even in mice, parasites were unable to form chronic stages when BFD2 was absent.
These findings initially did not make sense, because BFD2 appeared to control the same set of genes as BFD1. Licon had chosen to study BFD2 because it was directly regulated by BFD1; however, when she examined BFD1 expression in parasites lacking BFD2, she found that the expression of BFD1 was dependent on BFD2.
Licon proposed that BFD1 and BFD2 are engaged in positive feedback. At a molecular level, BFD2 is an RNA-binding protein, which she found was needed to make BFD1 protein from the messenger RNA. Like a chain reaction, a small amount of BFD2 will generate BFD1, which in turn helps Toxo make more BFD2. The cycle continues until enough BFD1 has been made to drive the transition to the chronic state.
“If a parasite is going to try and commit to being in a different state, it needs to avoid going backwards,” explained Lourido. “So, we’ve now found a positive feedback loop that provides momentum to keep the parasite in a certain state. It’s a nice way of understanding that once the parasite gets the signal to differentiate, it has this robust force to help drive it to the chronic stage and stay in the chronic stage.”
Although drugs active against the acute form of Toxo exist, the chronic stage is resistant to these drugs, as well as to our immune response. Toxo infections can therefore be managed with such drugs, but they cannot be cured. Understanding what drives the change between acute and chronic phases could ultimately lend insight into more effective treatment against Toxo. “In principle, I think that if Toxo can’t make these chronic stages, you might be able to clear it with drugs,” Licon said.
Until then, Licon continues to work on figuring out why BFD2 only functions under certain conditions. “We have our foot in the door,” she said, “and now we are going to keep following this pathway upwards in order to understand the higher complexity of what is going on.”
More information:
M. Haley Licon et al, A positive feedback loop controls Toxoplasma chronic differentiation, Nature Microbiology (2023). DOI: 10.1038/s41564-023-01358-2
Citation:
Positive feedback loop drives transition of Toxoplasma gondii to chronic stage (2023, April 21)
retrieved 21 April 2023
from https://phys.org/news/2023-04-positive-feedback-loop-transition-toxoplasma.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

