PDAT regulates phosphatidylethanolamine as transient carbon sink alternative to triacylglycerol in nannochloropsis
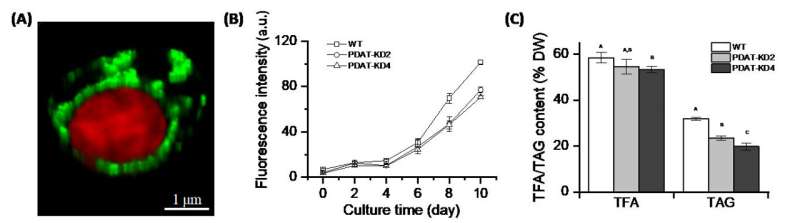
Nannochloropsis is a group of unicellular eukaryotes that belong to the class Eustigmatophyceae. Currently, there are seven identified species in this genus that have high photosynthetic efficiency, biomass and oil content (triacylglycerol, or TAG), and are rich in eicosapentaenoic acid (EPA), making them high-quality raw materials for the industrial production of EPA.
Nannochloropsis oceanica has been recognized as an industrially important single-cell factory for lipid production because of its fast growth and high lipid content. Its genome contains up to 13 acyl CoA:diacylglycerol (DAG) acyltransferases (NoDGATs), which contribute to stress and non-stress associated TAG biosynthesis, and raises questions about whether the unique N. oceanica phospholipid:DAG acyltransferase (NoPDAT) is critical, and under what conditions and to what extent it contributes to TAG biosynthesis.
A research team led by Prof. Hu Hanhua from the Institute of Hydrobiology (IHB) of the Chinese Academy of Sciences recently addressed the function and physiological role of NoPDAT in N. oceanica. The study was published in Plant Physiology.
Prof. Hu’s team has carried out a series of basic researches on the above-mentioned algal strains for more than ten years. They first established an efficient genetic transformation system based on polymerase chain reaction products and a gene knockdown system based on RNA interference in all the six marine species.
The researchers achieved the high-efficiency genetic transformation based on electroporation by chemical pretreatment in Nannochloropsis limnetica, the only freshwater alga in this genus.
They found that NoPDAT resides at the outermost plastid membrane, the chloroplast endoplasmic reticulum. Based on genetic analysis, NoPDAT contributes at least 30% to TAG biosynthesis under nitrogen-limited conditions, and NoPDAT knockdown has not triggered any compensatory mechanism via DGATs.
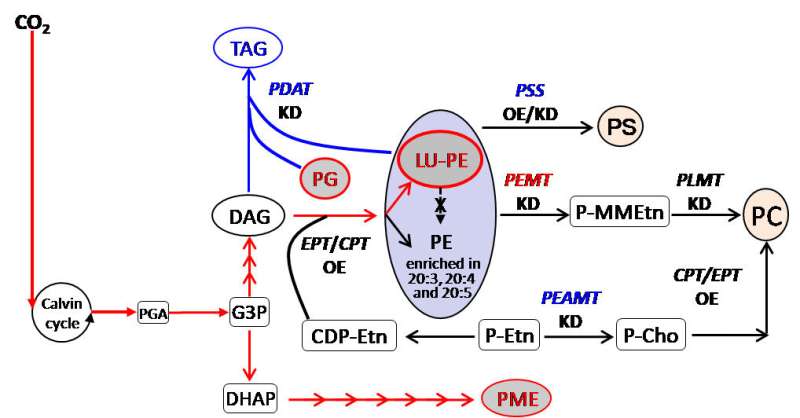
Through the semi-quantitative thin layer chromatography analysis of polar lipids, the researchers also found that NoPDAT knockdown leads to a vast accumulation of a new class of phosphatidylethanolamines (PEs) in cells, and this special PE containing 16:0, 16:1, and 18:1 fatty acids (referred to as “LU-PE”) differs from the intracellular functional PEs containing polyunsaturated fatty acids (C20:4 and C20:5).
Moreover, the content of intracellular LU-PE was significantly positively correlated with the carbon dioxide (CO2) concentration in culture.
Results of overexpressing and/or knockdown of genes involved in PE homeostasis indicated that LU-PE accumulation in N. oceanica is not linked to these genes and that NoPDAT is solely responsible for the observed profile change.
In summary, this study uncovers that the NoPDAT pathway is parallel to and independent of the NoDGAT pathway for oil production. LU-PE can serve as an alternative carbon sink for photosynthetically assimilated carbon in N. oceanica when PDAT-mediated TAG biosynthesis is compromised or under stress in the presence of high CO2 levels.
Scientists program microalgae’s ‘oil factory’ to produce various oils
Juan Yang et al, PDAT regulates PE as transient carbon sink alternative to triacylglycerol in Nannochloropsis, Plant Physiology (2022). DOI: 10.1093/plphys/kiac160
Citation:
PDAT regulates phosphatidylethanolamine as transient carbon sink alternative to triacylglycerol in nannochloropsis (2022, June 7)
retrieved 7 June 2022
from https://phys.org/news/2022-06-pdat-phosphatidylethanolamine-transient-carbon-alternative.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

