Meet the (protein) neighbors: New method lets researchers detect proteins in close proximity in single cells
Today, most methods to determine the proteins inside a cell rely on a crude census—scientists usually grind a large group of cells up before characterizing their genetic material. But just as a population of 100 single people differs in many ways from a population of 20 five-person households, this kind of description fails to capture information about how proteins are interacting and clumping together into functional groups.
Now, researchers at the University of Chicago’s Pritzker School of Molecular Engineering (PME) have developed an approach that lets them more easily study whether proteins are located in close proximity to each other inside a cell. The technology, which can be carried out at the same time as more routine gene sequencing, is described in the journal Nature Methods.
“This is a streamlined and high-throughput way to look into protein functions inside individual cells,” said Savas Tay, professor of molecular engineering and senior author of the new work. “I think this method is going to be a major resource for the molecular biology community.”
Focused on function
In recent years, with the advent of fast and cheap genetic sequencing technologies, scientists have turned to single-cell mRNA sequencing to get snapshots of cell’s internal states. By sequencing all of a cell’s mRNA molecules—which encode proteins—they can get an idea of what proteins a cell might be actively using. But this kind of proxy for protein abundance doesn’t convey the full story.
“Just knowing the abundance of certain proteins doesn’t always give you information about how a cell is functioning,” said Tay. “Often, whether or not proteins are functionally active has to do with not just whether they are present but whether they are forming complexes.”
Tay wanted to capture whether proteins were physically near each other—and do so in a fast, high-throughput way, not by relying on microscopy to visualize their locations or isolating a few proteins at a time for closer inspection.
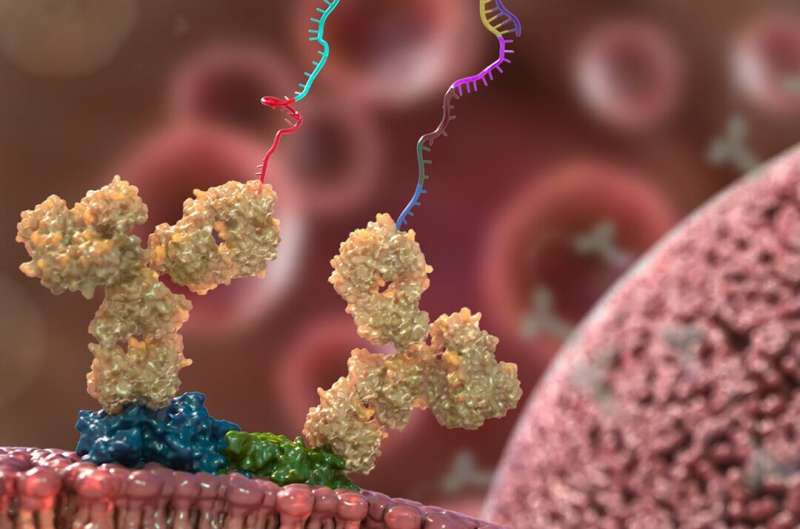
He and his colleagues developed molecular probes that attach to proteins of interest and have DNA tags extending outward. If two proteins are physically close to each other, those DNA probes stick together like Velcro. The researchers can set up experiments that simultaneously probe hundreds of proteins in this way. Then, they use routine sequencing methods to read back any DNA probes that bound to each other and determine which proteins were paired up.
“In the vast space inside a cell, it’s very unlikely for two probes to find each other unless they are bound to nearby proteins,” said Tay. “So when the probes bind, that tells us these proteins are very close together.”
Since the approach, dubbed Prox-seq, uses standard sequencing, researchers can analyze a cell’s mRNA at the same time as the proteins of interest, to help answer questions about the correlation between mRNA and protein abundance and function.
Proof-of-concept studies
To illustrate the utility of Prox-seq, Tay’s team first tested it on B cells and T cells, two subsets of human immune cells. The technique, they found, could differentiate the cells based solely on which protein-protein interactions were present in each cell type. It also could identify previously unknown subsets of the cells based on small differences in how groups of proteins were organized within the cells. Using human derived peripheral blood mononuclear cells, they simultaneously measured 38 individual proteins, 741 protein complexes, and thousands of mRNA on each individual cell, and discovered a new protein complex that defines naïve T cells.
Then, the group used Prox-seq to study how proteins change arrangement in macrophages, another type of immune cell, when the cells become activated in response to a pathogen. Once again, they identified both known pairs of interacting proteins as well as new groups of proteins that can be used to identify not only whether a macrophage has been activated but also what type of pathogen it was exposed to.
“This showed that not only can our method verify protein-protein complexes that we already knew about, but it can find new interactions between proteins,” said Tay.
So far, the researchers have only tested the method on proteins found on the surface of cells; they’re now working to expand it to more types of proteins as develop ways to resolve exactly where within a cell proteins are interacting.
More information:
Luke Vistain et al, Quantification of extracellular proteins, protein complexes and mRNAs in single cells by proximity sequencing, Nature Methods (2022). DOI: 10.1038/s41592-022-01684-z
Citation:
Meet the (protein) neighbors: New method lets researchers detect proteins in close proximity in single cells (2022, December 6)
retrieved 6 December 2022
from https://phys.org/news/2022-12-protein-neighbors-method-proteins-proximity.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

