Commonly used chemical fixation causes aggregation artifact
Researchers at Kanazawa University report in Communications Biology that using common chemicals for fixing living cell samples for microscopy studies causes membrane proteins to aggregate.
For histological investigations of biological tissues, i.e. anatomical studies under the microscope, samples are usually fixated to prevent them from decaying. Fixation is typically done by immersing or perfusing the sample in a chemical—aldehydes and alcohols are common fixatives. It has been speculated that membrane proteins moving to some extent on a cell membrane can form aggregates during fixation. Yet, detailed cell surface studies with the nanometer-scale resolution are necessary for obtaining definitive insights into this potential issue. Now, Takehiko Ichikawa and colleagues from Kanazawa University have performed atomic force microscopy (AFM) studies of living mammalian cell surfaces. By comparing non-fixated and fixated samples, they found that fixation indeed leads to structural changes.
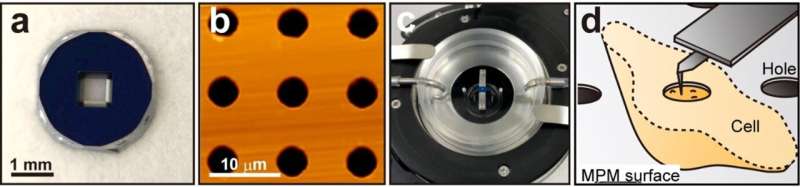
The researchers developed a method of using microporous silicon nitride membrane (MPM), used in transmission electron microscopy (Figure 1), for AFM imaging. Importantly, MPM can make the cell surface flat and prevent fluctuations by supporting the area outside the observation area. In AFM images of the surfaces of the cultured colon cancer cells on MPM, biomolecular structures on the cell membranes showed up as protrusions with a typical size of a few nanometers (Figure 2 living cell surface).
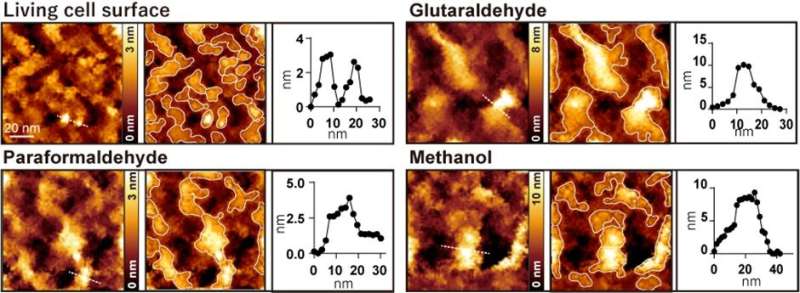
When the cells were treated with commonly used fixatives such as paraformaldehyde, glutaraldehyde, and methanol, a few nanometer structures disappeared, and only large protrusions with diameters ranging from 20 to 100 nanometers were observed (Figure 2). The researchers performed several fluorescence experiments and concluded that large protrusions observed after fixation were formed by the aggregation of membrane proteins.
The study demonstrates that the observed aggregates are artifacts resulting from the fixation process. This should call for caution among the community of researchers working with chemical fixatives. Quoting Ichikawa and colleagues: “Researchers who observe nanoscale clusters also should be careful in interpreting their experimental results when using fixed cells. We recommend that researchers use living cells as much as possible to avoid the effect of fixation when investigating nanoscale clusters.”
The general principle behind atomic force microscopy (AFM) is to make a very small tip scan the surface of a sample. During this horizontal (xy) scan, the tip, which is attached to a small cantilever, follows the sample’s vertical (z) profile, inducing a force on the cantilever that can be measured. The magnitude of the force at the xy position can be related to the z value; the xyz data generated during a scan then result in a height map providing structural information about the investigated sample. AFM is not affected by the diffraction limit due to the use of light or electron beams and can observe the intact surface topography with high resolution.
Assigning moving features in high-speed atomic force microscopy
Takehiko Ichikawa et al, Chemical fixation creates nanoscale clusters on the cell surface by aggregating membrane proteins, Communications Biology (2022). DOI: 10.1038/s42003-022-03437-2
Citation:
Commonly used chemical fixation causes aggregation artifact (2022, August 10)
retrieved 10 August 2022
from https://phys.org/news/2022-08-commonly-chemical-fixation-aggregation-artifact.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
For all the latest Science News Click Here
For the latest news and updates, follow us on Google News.

